The molecular velocities of two gases at same temperature are u1 and u2, their masses are m1 and m2 respectively, which of the following expression is correct ?
m1u21=m2u2
m1u1=m2u2
m1u1=m2u2
m1u21=m2u22
To solve the problem regarding the molecular velocities of two gases at the same temperature, we will derive the relationship between their masses and velocities step by step.
Step 1: Understand the RMS Velocity Formula
The root mean square (RMS) velocity of a gas is given by the formula:
u=√3RTm
where u is the RMS velocity, R is the universal gas constant, T is the temperature, and m is the molar mass of the gas.
Step 2: Write the RMS Velocity Equations for Both Gases
For gas 1 with mass m1 and velocity u1:
u1=√3RTm1
Squaring both sides, we get:
u21=3RTm1
(Equation 1)
For gas 2 with mass m2 and velocity u2:
u2=√3RTm2
Squaring both sides, we have:
u22=3RTm2
(Equation 2)
Step 3: Express Temperature in Terms of Velocities and Masses
From Equation 1, we can express T:
T=m1u213R
(Equation 3)
From Equation 2, we can also express T:
T=m2u223R
(Equation 4)
Step 4: Set the Two Expressions for Temperature Equal to Each Other
Since both gases are at the same temperature, we can equate Equation 3 and Equation 4:
m1u213R=m2u223R
Step 5: Simplify the Equation
The 3R cancels out from both sides:
m1u21=m2u22
Conclusion
The correct expression relating the masses and molecular velocities of the two gases is:
m1u21=m2u22
Final Answer
This means that the relationship between the molecular velocities and masses of the two gases is given by the equation above.
---
The root mean square (RMS) velocity of a gas is given by the formula:
u=√3RTm
where u is the RMS velocity, R is the universal gas constant, T is the temperature, and m is the molar mass of the gas.
For gas 1 with mass m1 and velocity u1:
u1=√3RTm1
Squaring both sides, we get:
u21=3RTm1
(Equation 1)
u2=√3RTm2
Squaring both sides, we have:
u22=3RTm2
(Equation 2)
From Equation 1, we can express T:
T=m1u213R
(Equation 3)
T=m2u223R
(Equation 4)
Since both gases are at the same temperature, we can equate Equation 3 and Equation 4:
m1u213R=m2u223R
The 3R cancels out from both sides:
m1u21=m2u22
The correct expression relating the masses and molecular velocities of the two gases is:
m1u21=m2u22
This means that the relationship between the molecular velocities and masses of the two gases is given by the equation above.
Topper's Solved these Questions
- STATES OF MATTERBook:AAKASH INSTITUTE ENGLISHChapter:STATES OF MATTERExercise:ASSIGNMENT (SECTION-C)
Explore 29 Videos
- STATES OF MATTERBook:AAKASH INSTITUTE ENGLISHChapter:STATES OF MATTERExercise:ASSIGNMENT (SECTION-D)
Explore 14 Videos
- STATES OF MATTERBook:AAKASH INSTITUTE ENGLISHChapter:STATES OF MATTERExercise:ASSIGNMENT (SECTION-A)
Explore 50 Videos
- SOME BASIC CONCEPTS OF CHEMISTRY Book:AAKASH INSTITUTE ENGLISHChapter:SOME BASIC CONCEPTS OF CHEMISTRY Exercise:ASSIGNMENT SECTION J (Aakash Challenges Questions)
Explore 10 Videos
- STRUCTURE OF ATOMBook:AAKASH INSTITUTE ENGLISHChapter:STRUCTURE OF ATOMExercise:ASSIGNMENT (SECTION -D) Assertion-Reason Type Questions
Explore 15 Videos
Similar Questions
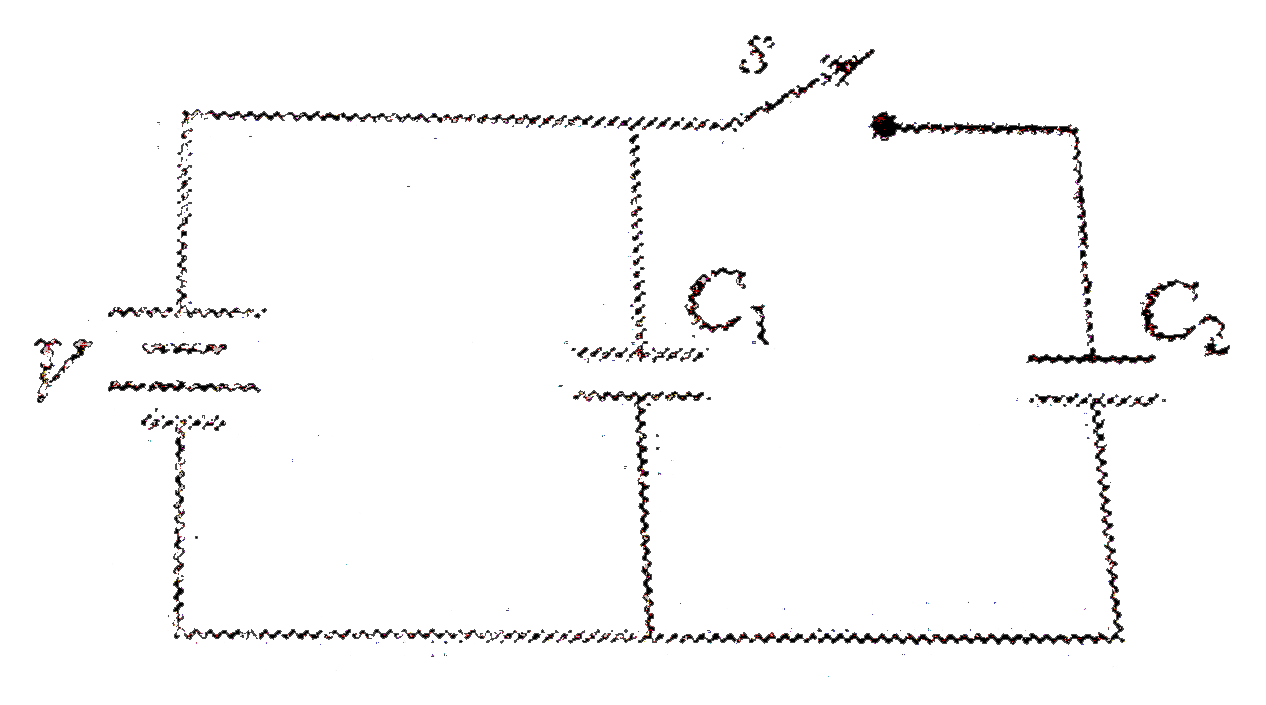
Two capacitors of equal capacitance (C1=C2) are as shown in the figure. Initially, while the swithc is open (as shown) one of the capacitors is uncharged and the other carries charge Q0. The energy stored in the charged capacitor is U0. Sometime after the switch is closed, the capacitors C1andC2 carry charged Q1andQ2 respectively, the energy stored in the capacitors are U1andU2 respectively. Which of the following expression is correct?
View SolutionThe lateral magnification of the lens with an object located at two different position u1 and u2 are m1 and m2 , respectively. Then the focal length of the lens is
View Solution
Knowledge Check
Two particles are projected simultaneously in the same vertical plane from the same point, with different speeds u1 and u2, making angles θ1 and θ2 respectively with the horizontal, such that u1cosθ1=u2cosθ2. The path followed by one, as seen by the other (as long as both are in flight), is
Aa vertical lineBa parabolaCa hyperbolaDa straight line making a constant angle (≠90∘) with horizontal
Two identical containers A and B having same volume of an ideal gas at same temperature have mass of the gas as m1 and m2 respectively and 2m1=3m2. The gas in each cylinder expands isomthermally to double of its volume. If change in pressure in A is 300Pa, then the change in pressure in B is
View SolutionTwo perfect gases (1) and (2) are at temperatures T1 and T2 . If the number of molecules of the two gases are n1 and n2 and the masses of the molecules m1 and m2, find the resulting temperature when the two gases are mixed. Assume that there is no loss of energy on mixing the two gases.
View SolutionTwo particles are projected simultaneously in the same vertical plane, with speed u1 and u2 at angle of projection θ1 and θ2 respectively with the horizontal. The path followed by one, as seen by other (as long as both are in flight), is
View SolutionTwo perfect gases at absolute temperature T1andT2 are mixed. There is no loss of energy. The masses of the molecules are m1andm2. The number of molecules in the gases are n1andn2. The temperature of the mixture is
View SolutionTwo concentric shells of masses M1 and M2 are having radii r1 and r2. Which of the following is the correct expression for the gravitational field on a mass m?

View SolutionTwo concentric shells of masses M1 and M2 are having radii r1 and r2. Which of the following is the correct expression for the gravitational field on a mass m?

View SolutionThe numerical values and units of a physical quantity in two different system of units are n1,n2andu1,u2 respectively. Then
View Solution
