Among the following order of energies of molecular orbitals of O2 choose the correct one
E(π2px)=E(π2py)<E(σ2pz)<(Eπ∗2px)=E(π∗2±py)
E(π2px)=E(π2py)>E(σ2pz)>(Eπ∗2px)=E(π∗2py)
E(π2px)=E(π2py)<E(σ2pz)>(Eπ∗2px)=E(π∗2py)
E(π2px)=E(π2py)>E(σ2pz)<(Eπ∗2px)=E(π∗2py)
The correct Answer is:D
To determine the correct order of energies of molecular orbitals in O2, we will follow these steps:
Step 1: Understand the Molecular Orbital Theory
Molecular Orbital (MO) theory explains how atomic orbitals combine to form molecular orbitals. In diatomic molecules, the atomic orbitals of the constituent atoms combine to form bonding and antibonding molecular orbitals.
Hint: Familiarize yourself with the concept of bonding and antibonding orbitals.
Step 2: Identify the Atomic Orbitals of Oxygen
Oxygen has the electronic configuration of 1s² 2s² 2p⁴. For O2, we will focus on the 2p orbitals, which are 2px, 2py, and 2pz.
Hint: Remember that each oxygen atom contributes its atomic orbitals to form molecular orbitals.
Step 3: Draw the Molecular Orbital Diagram for O2
In the molecular orbital diagram for O2, the order of energy for the 2p molecular orbitals is as follows:
- σ2pz (lowest energy)
- π2px = π2py (higher energy, degenerate)
- π*2px = π*2py (higher energy, degenerate)
- σ*2pz (highest energy)
Hint: Pay attention to the relative energies of the bonding and antibonding orbitals.
Step 4: Establish the Energy Order
From the molecular orbital diagram, we can establish the energy order:
1. σ*2pz (highest energy)
2. π*2px = π*2py
3. π2px = π2py
4. σ2pz (lowest energy)
Hint: Note that bonding orbitals are lower in energy than antibonding orbitals.
Step 5: Analyze the Given Options
Now, we will analyze the options provided in the question:
1. Option 1 states π2px and π2py have equal energy, which is less than σ2pz (incorrect).
2. Option 2 states π2px equals π2py, which is greater than σ2pz (incorrect).
3. Option 3 states energy of 2px equals energy of 2py, which is less than σ2pz (incorrect).
4. Option 4 states π2px equals π2py, which is greater than σ2pz (correct).
Hint: Compare each option with the established energy order to identify the correct one.
Conclusion
The correct order of energies of molecular orbitals for O2 is represented in option 4.
Final Answer: Option 4 is the correct choice.
---
Molecular Orbital (MO) theory explains how atomic orbitals combine to form molecular orbitals. In diatomic molecules, the atomic orbitals of the constituent atoms combine to form bonding and antibonding molecular orbitals.
Oxygen has the electronic configuration of 1s² 2s² 2p⁴. For O2, we will focus on the 2p orbitals, which are 2px, 2py, and 2pz.
In the molecular orbital diagram for O2, the order of energy for the 2p molecular orbitals is as follows:
- σ2pz (lowest energy)
- π2px = π2py (higher energy, degenerate)
- π*2px = π*2py (higher energy, degenerate)
- σ*2pz (highest energy)
From the molecular orbital diagram, we can establish the energy order:
1. σ*2pz (highest energy)
2. π*2px = π*2py
3. π2px = π2py
4. σ2pz (lowest energy)
Now, we will analyze the options provided in the question:
1. Option 1 states π2px and π2py have equal energy, which is less than σ2pz (incorrect).
2. Option 2 states π2px equals π2py, which is greater than σ2pz (incorrect).
3. Option 3 states energy of 2px equals energy of 2py, which is less than σ2pz (incorrect).
4. Option 4 states π2px equals π2py, which is greater than σ2pz (correct).
The correct order of energies of molecular orbitals for O2 is represented in option 4.
Topper's Solved these Questions
Similar Questions
Knowledge Check
Which of the following orderof energies of molecular orbitals of N2 is correct?
A(π2py)<(σ2pz)<(π⋆2px)≈(π⋆2py)B(π2py)>(σ2pz)>(π⋆2px)≈(π⋆2py)C(π2py)<(σ2pz)>(π⋆2px)≈(π⋆2py)D(π2py)>(σ2pz)<(π⋆2px)≈(π⋆2py)Which of the following representation of wave functions of molecular orbitals and atomic orbitals are not correct ?
AΨMO=ΨA±ΨBBσ=ΨA+ΨBCσ⋆=ΨA−ΨBDΨMO=ΨA×ΨB
Which among the following order of given properties is correct
View SolutionThe energies of orbitals of hydrogen atom are in the order
View SolutionAmong the following, the correct order of acidity is:
View SolutionAmong the following
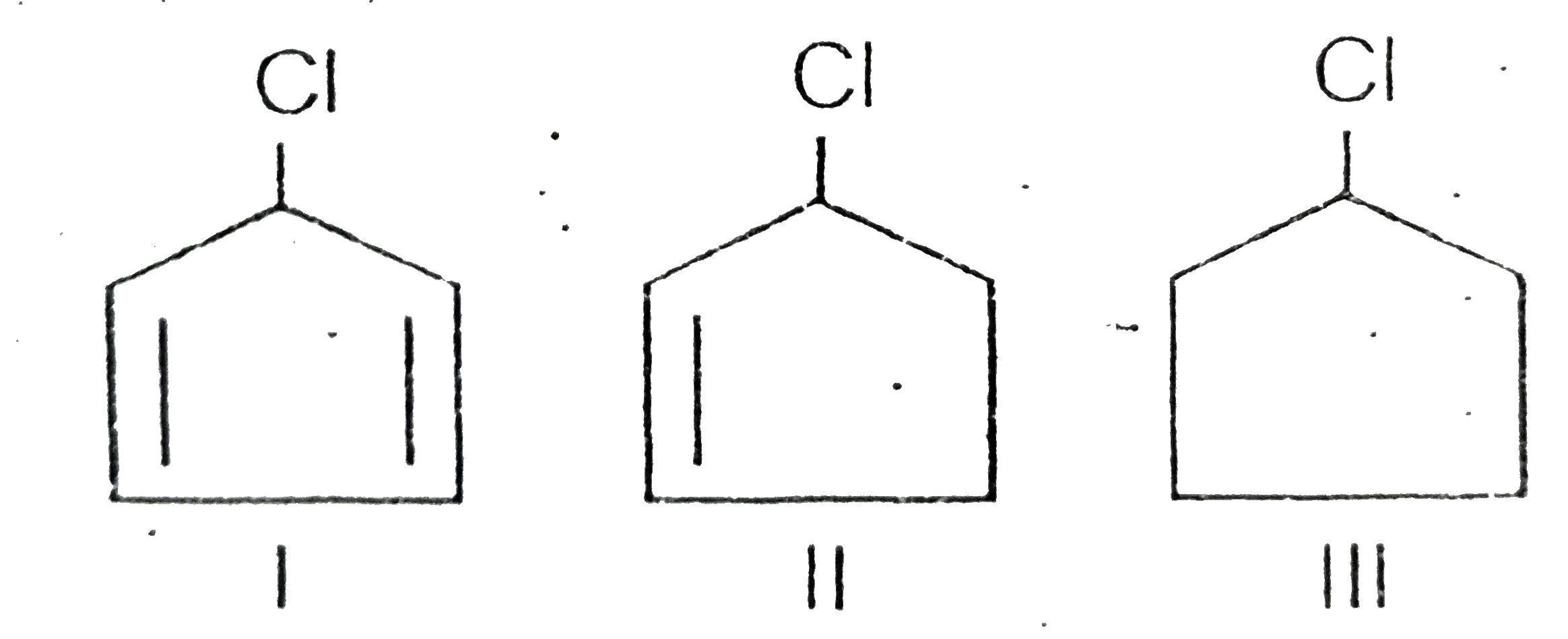
The correct order of reactivity of chloride is
View SolutionWhich of the following order of molecular force of attraction among given species is incorect ?
View SolutionWhich of the following order of molecular force of attraction among given species is incorect ?
View Solution

