In which of the following alkane,isomerisation will not occur?
C2H6
C4H10
C5H10
C6H14
To determine which alkane will not undergo isomerization, we need to understand the concept of isomerization and the structural requirements for it to occur.
1. Understanding Isomerization:
- Isomerization is a process where a compound can rearrange its structure to form different isomers. Isomers have the same molecular formula but different structural arrangements.
2. Identifying the Minimum Carbon Requirement:
- For alkanes, isomerization generally requires a minimum of 4 carbon atoms. This is because with fewer than 4 carbons, there are not enough structural variations possible to create isomers.
3. Analyzing the Given Alkanes:
- C2H6 (Ethane): This alkane has only 2 carbon atoms. Since there are no structural variations possible with just 2 carbons, isomerization cannot occur.
- C3H8 (Propane): This alkane has 3 carbon atoms. Similar to ethane, there are no structural variations possible, so isomerization cannot occur.
- C4H10 (Butane): This alkane has 4 carbon atoms. It can exist as two isomers: n-butane and isobutane (or methylpropane), indicating that isomerization can occur.
- C5H12 (Pentane): This alkane has 5 carbon atoms and can have multiple structural isomers (n-pentane, isopentane, and neopentane), so isomerization can occur.
- C6H14 (Hexane): This alkane has 6 carbon atoms and can also form several isomers, indicating that isomerization can occur.
4. Conclusion:
- The only alkane from the options provided that cannot undergo isomerization is C2H6 (Ethane), as it does not have enough carbon atoms to form different structural isomers.
Final Answer:
The alkane in which isomerization will not occur is C2H6 (Ethane).
- Isomerization is a process where a compound can rearrange its structure to form different isomers. Isomers have the same molecular formula but different structural arrangements.
- For alkanes, isomerization generally requires a minimum of 4 carbon atoms. This is because with fewer than 4 carbons, there are not enough structural variations possible to create isomers.
- C2H6 (Ethane): This alkane has only 2 carbon atoms. Since there are no structural variations possible with just 2 carbons, isomerization cannot occur.
- C3H8 (Propane): This alkane has 3 carbon atoms. Similar to ethane, there are no structural variations possible, so isomerization cannot occur.
- C4H10 (Butane): This alkane has 4 carbon atoms. It can exist as two isomers: n-butane and isobutane (or methylpropane), indicating that isomerization can occur.
- C5H12 (Pentane): This alkane has 5 carbon atoms and can have multiple structural isomers (n-pentane, isopentane, and neopentane), so isomerization can occur.
- C6H14 (Hexane): This alkane has 6 carbon atoms and can also form several isomers, indicating that isomerization can occur.
- The only alkane from the options provided that cannot undergo isomerization is C2H6 (Ethane), as it does not have enough carbon atoms to form different structural isomers.
The alkane in which isomerization will not occur is C2H6 (Ethane).
Topper's Solved these Questions
Similar Questions
Knowledge Check
In which of the following taxa does photosynthesis not occur ?
Aa) EuglenophytaBb) PyrrophytaCc) RhodophytaDd) Sporozoa
Which of the following halogen exchange reaction will occur in acetone ?
View SolutionWhich of the following alkane has longest Carbon chain :
View SolutionWhich of the following alkane cannot show isomerism?
View SolutionIn which of the following diseases keratinization occurs in cornea ?
View SolutionIn which of the following plant, pollination occurs by the act of pseudocopulation?
View SolutionWhich of the following does not occur?
View SolutionIn which of the following steps does inversion occur?
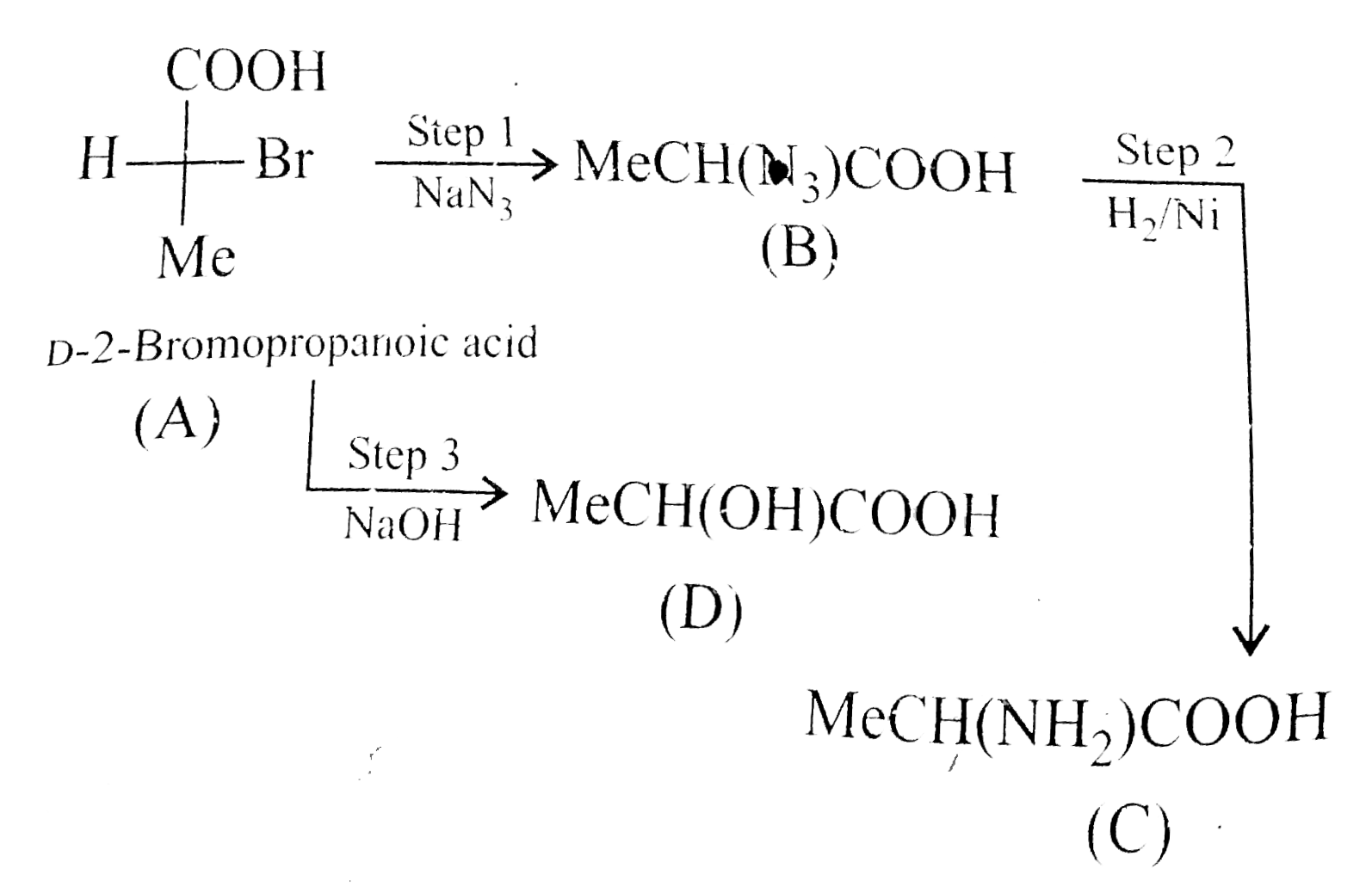
View Solution
