Observe the following compounds
(A) CH3CH2CHO
(B) CH3COCH3
(C) Ph-CHO
(D)CH3CH(CH3)CHO
(E) HCHO
(A),(B), and (C) will give aldol condensation reaction
(A),(B) and (D) will give aldol condensation reaction
(B),(D) and (E) wil give Cannizzaro reaction
(C) and (E) will give Cannizzaro reaction
To solve the question regarding the compounds and their reactions, we will analyze each compound to determine whether they can undergo aldol condensation or Cannizzaro reaction based on the presence of alpha hydrogen atoms.
1. Identify Compound A: CH3CH2CHO
- Structure: This compound is an aldehyde with the structure CH3-CH2-CHO.
- Analysis: The carbon adjacent to the carbonyl (CHO) has two hydrogen atoms (the CH2 group). Therefore, it has alpha hydrogens.
- Conclusion: This compound can undergo aldol condensation.
2. Identify Compound B: CH3COCH3
- Structure: This compound is a ketone with the structure CH3-CO-CH3.
- Analysis: The carbonyl carbon (C=O) has two adjacent carbons (both CH3 groups). Each of these carbons has three hydrogen atoms.
- Conclusion: This compound can undergo aldol condensation.
3. Identify Compound C: Ph−CHO
- Structure: This compound is benzaldehyde, with the structure Ph-CHO (where Ph represents a phenyl group).
- Analysis: The carbon adjacent to the carbonyl carbon (CHO) is part of the phenyl ring, which does not have any hydrogen atoms attached to it.
- Conclusion: This compound cannot undergo aldol condensation (it will undergo Cannizzaro reaction instead).
4. Identify Compound D: CH3CH(CH3)CHO
- Structure: This compound is an aldehyde with the structure CH3-CH(CH3)-CHO.
- Analysis: The carbon adjacent to the carbonyl carbon has one hydrogen atom (the CH group) and is attached to two other carbons (one CH3 and one CH).
- Conclusion: This compound can undergo aldol condensation.
5. Identify Compound E: HCHO
- Structure: This compound is formaldehyde.
- Analysis: There is only one carbon atom in this compound, and it has no adjacent carbon (no alpha carbon).
- Conclusion: This compound cannot undergo aldol condensation (it will undergo Cannizzaro reaction instead).
Summary of Reactions:
- Aldol Condensation: Compounds A, B, and D have alpha hydrogens.
- Cannizzaro Reaction: Compounds C and E do not have alpha hydrogens.
Final Answer:
- Option 2: A, B, and D will give aldol condensation reaction.
- Option 4: C and E will give Cannizzaro reaction.
- Structure: This compound is an aldehyde with the structure CH3-CH2-CHO.
- Analysis: The carbon adjacent to the carbonyl (CHO) has two hydrogen atoms (the CH2 group). Therefore, it has alpha hydrogens.
- Conclusion: This compound can undergo aldol condensation.
- Structure: This compound is a ketone with the structure CH3-CO-CH3.
- Analysis: The carbonyl carbon (C=O) has two adjacent carbons (both CH3 groups). Each of these carbons has three hydrogen atoms.
- Conclusion: This compound can undergo aldol condensation.
- Structure: This compound is benzaldehyde, with the structure Ph-CHO (where Ph represents a phenyl group).
- Analysis: The carbon adjacent to the carbonyl carbon (CHO) is part of the phenyl ring, which does not have any hydrogen atoms attached to it.
- Conclusion: This compound cannot undergo aldol condensation (it will undergo Cannizzaro reaction instead).
- Structure: This compound is an aldehyde with the structure CH3-CH(CH3)-CHO.
- Analysis: The carbon adjacent to the carbonyl carbon has one hydrogen atom (the CH group) and is attached to two other carbons (one CH3 and one CH).
- Conclusion: This compound can undergo aldol condensation.
- Structure: This compound is formaldehyde.
- Analysis: There is only one carbon atom in this compound, and it has no adjacent carbon (no alpha carbon).
- Conclusion: This compound cannot undergo aldol condensation (it will undergo Cannizzaro reaction instead).
- Aldol Condensation: Compounds A, B, and D have alpha hydrogens.
- Cannizzaro Reaction: Compounds C and E do not have alpha hydrogens.
- Option 2: A, B, and D will give aldol condensation reaction.
- Option 4: C and E will give Cannizzaro reaction.
Topper's Solved these Questions
Similar Questions
Ethylamine is soluble in water whereas aniline is almost insoluble. Why?
View SolutionIf helium and oxygen gas are allowed to escape out of a container containing equal amount of He and O2 under slmllar conditions of temperature and pressure the ratio of rate of diffusion of O2 to that of He is
View SolutionConvert CH_(3)CHO into the following compounds: a. b. c. d. e.
View SolutionWhich of the following undergoes cannizaro reaction ? A)HCHO B) C_(6)H_(5)CHO C) Cl_(3)C-CHO D) (CH_(3))_(3)C-CHO
View SolutionOne mole of an unsaturated hydrocarbons on ozonolysis gives one mole each of CH3CHO,HCHO and OHC−CHO. The hydrocarbon is:
View Solutionनामकरण की IUPAC पध्दति के अनुसार निम्न यौगिकों के नाम बताइए -
(a) CH3CH(CH3)CH2CH2CHO
(b) CH3CH2COCH(C2H5)CH2CH2Cl
(c) CH3CH=CHCHO
(d)CH3COCH2COCH3
(e) CH3CH(CH3)CH2C(CH3)2COCH3
(f) (CH3)3CCH2COOH
(g) OHCC6H4CHO(−p) .
View SolutionA1.O3−−−−−−→2.Zn,H2O(CH3)2CO+HCHO+CHO∣∣∣CHO Thus A is :
View SolutionHow many of these compounds can undergo cannizzaro reaction here
CH3CHO,CH3COCH3,HCHO,Ph−CHO,Ph−CO−CHO,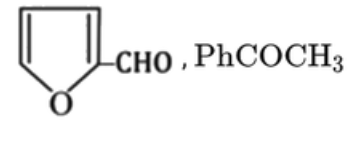 , PhCOCH3
, PhCOCH3
View SolutionHow many of these compounds can undergo Cannizzaro reaction here
CH3CHO,CH3COCH3,HCHO,Ph−CHO,Ph−CO−CHO,
 , PhCOCH3
, PhCOCH3
View SolutionCH_3CH(CHO)-CH(CH_2CH_3)C(CH_3)_2CH(CHO)CH(CH_2CH_3)_2 Name the formula in IUPAC?
View SolutionCH3CHO+CH3CH2CHONaOH.Δ−−−−−→
Which one of the following compounds is not the product in the above reaction?
View SolutionWrite the reactions between ammonia with
(i) CH3CHO and (ii) CH3COCH3.
View SolutionThe compound CH_3CH_2CHO and CH_3COCH_3 illustrate:
View SolutionObserve the following compounds
(A) CH3CH2CHO
(B) CH3COCH3
(C) Ph−CHO
(D)CH3CH(CH3)CHO
(E) HCHO
View SolutionIdentify the isomerism exhibited by the following compounds. CH_3CH_2CHO and CH_3COCH_3
View Solution

