Balance the following equation by the oxidation number method.
Cr(s)+Pb(NO3)2(aq)→Cr(NO3)3(aq)+Pb(s)Solution in Telugu
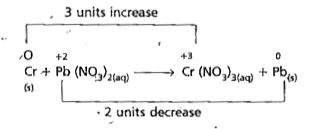
So the balanced equation is 2Cr + 3Pb (NO3)2→2Cr(NO3)3+3Pb
Topper's Solved these Questions
- STOICHIOMETRY BOOK - VIKRAM PUBLICATION ( ANDHRA PUBLICATION)CHAPTER - STOICHIOMETRY EXERCISE - SHORT ANSWER QUESTIONS
88 Videos
- STOICHIOMETRY BOOK - VIKRAM PUBLICATION ( ANDHRA PUBLICATION)CHAPTER - STOICHIOMETRY EXERCISE - LONG ANSWER QUESTIONS
6 Videos
- STOICHIOMETRY BOOK - VIKRAM PUBLICATION ( ANDHRA PUBLICATION)CHAPTER - STOICHIOMETRY EXERCISE - Important Question
39 Videos
- STATES OF MATTER GASES AND LIQUIDS BOOK - VIKRAM PUBLICATION ( ANDHRA PUBLICATION)CHAPTER - STATES OF MATTER GASES AND LIQUIDS EXERCISE - LONG ANSWER QUESTIONS
22 Videos
- THE P-BLOCK ELEMENTS GROUP-13BOOK - VIKRAM PUBLICATION ( ANDHRA PUBLICATION)CHAPTER - THE P-BLOCK ELEMENTS GROUP-13EXERCISE - Long Answer Questions
3 Videos
Similar Questions
The approximate production of sodium carbonate per month is 424×106g. While that of methyl alcohol is 320×106 gm. Which is produced more in terms of moles ?
View SolutionWrite the balanced ionic equation which represents the oxidation of iodine (I−) ion by per-manganate ion in basic medium to give iodine (I) and manganese dioxide (MnO2).
View SolutionHow many number of moles of glucose are present in 540 gms of glucose ?
View SolutionWrite notes on Intermolecular forces
View SolutionHow are borax and boric acid prepared ? Explain the action of heat on them.
View SolutionWrite balanced ionoic half equation (oxidation and reduction) for each of the following reactions: (a) Mn^(3+)(aq)rarrMnO_(2)(s)+Mn^(2+)(aq) (b) Mn(s)+NO_(3)^(Ө)(aq) rarr Mn^(2+)(aq)+NO_(2)(g) (c ) H_(2)O_(2)(aq) + Fe^(2+) (aq)rarrFe^(3+)(aq)+H_(2)O(l) (d) Te(s)+NO_(3)^(Ө)(aq)rarr TeO_(2)(s)+NO(g)
View SolutionBalance the following equation by oxidation number method Zn+HNO_(3) to Zn(NO_(3))_(2)+NH_(4)NO_(3)+H_(2)
View Solutionनिम्नलिखित समीकरण को ऑक्सीकरण संख्या विधि के द्वारा संतुलित कीजिए-
Zn+HNO3→Zn(NO3)2+NH4NO3+H2O
View SolutionBalance the equation
Mg+HNO3→Mg(NO3)2+N2O+H2O
View SolutionBalance the equation As_(2)(s)+NO_(3)^-)(aq)+H^(+)(aq)rarrAsO_+(4)^(3-)(aq)+S(s)NO(g)+H_(2)O(l)
View SolutionBalance the equation Mg(aq)+HNO_(3)(aq)rarrMg(NO_(3))_(2)(aq)+N_(2)O(g)+H_(2)O(l)
View SolutionBalance the equation
As2(s)+NO−3(aq)+H+(aq)→AsO+(4)3−(aq)+S(s)NO(g)+H2O(l)
View SolutionBalance the following equation in acidic medium by boht oxidation numbr and ion electron methods nad identify the oxidatns and the reductants (i) MnO_(4)^(-)(aq)+C_(2)H_(2)O_(4)(aq) to Mn^(2+)(aq)+CO_(2)(g)+H_(2)O(l) (ii) H_(2)S(aq)+CI_(2)(g) to S(s)+CI^(-)(aq) (iii) MnO_(4)^(-)(aq)+C_(2)H_(5)OH(aq) to Mn^(2+)(aq)+CH_(3)COOH(aq) (iv) Bi(s)+NO_(3)^(-)(aq) to Bi^(3+)(aq)+NO_(2)(g) (v) Cr_(2)O_(7)^(2-)(aq)+c_(2)H_(4)O(aq)to Cr^(3+)(aq)+C_(2)H_(4)O_(2)(aq) (vi) MnO_(4)^(-)(q)+Br^(-)(aq) to Mn^(2+)(q)+Br_(2)(aq) (vii) Cu(Aq)+NO_(3)^(-)(aq) to Cu^(2+)(aq)+NO_(2)(g) (viii) H_(2)S(g)+Fe^(3+)(aq) to Fe^(2+)(aq)+S(s)+H^(+)(aq) (ix) I^(-)(aq)+O_(2)(g)+H_(2)O(l) to I_(2)(aq)+(OH)^(-)(aq) (x) Zn(s)+NO_(3)^(-)(aq)+H^(+)(aq) to Zn^(2)(aq)+N_(2)O(g)+H_(2)O(l) (xi) As(s)+NO_(3)^(-)(aq)+H^(+)(aq) to AsO_(3)^(3-)(aq)+NO_(2)(g)+H_(2)O(l) (xii) MnO_(4)^(-)(aq)+Fe^(2+)(aq) to Mn^(2+)(aq)+Fe^(3)(aq) (xiii) S+HNO_(3) to SO_(2)+NO_(2)+H_(2)O
View SolutionBalance the following chemical equations.
i) Zn(s)+AgNO3(aq)→Zn(NO3)2(aq)+Ag(s)
View SolutionBalance the following equation by the oxidation number method.
Cr(s)+Pb(NO3)2(aq)→Cr(NO3)3(aq)+Pb(s)
View SolutionAmong the reactions given the oxidant and the reductant in each case: (NH_(4))_(2)S(aq)+Cu(NO_(3))_(2)(aq)toCuS(s)+2NH_(4)NO_(3)(aq)
View Solution

