A carbon compound on analysis gave the following percentage composition, carbon 14.5%, hydrogen 1.8%, chlorine 64.46%, oxygen 19.24%. Calculate the empirical formula of the compound.Solution in Telugu
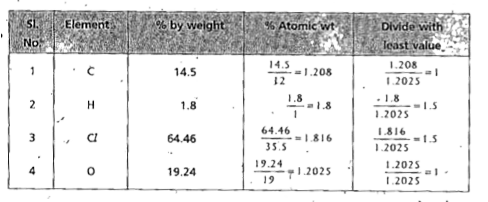 :. The formula of the compound = C1H1.5Cl1.5O1
:. The formula of the compound = C1H1.5Cl1.5O1
Emperical formula of the compound = C2H3Cl3O2.
Topper's Solved these Questions
- STOICHIOMETRY Book:VIKRAM PUBLICATION ( ANDHRA PUBLICATION)Chapter:STOICHIOMETRY Exercise:LONG ANSWER QUESTIONS
Explore 6 Videos
- STOICHIOMETRY Book:VIKRAM PUBLICATION ( ANDHRA PUBLICATION)Chapter:STOICHIOMETRY Exercise:Important Question
Explore 39 Videos
- STOICHIOMETRY Book:VIKRAM PUBLICATION ( ANDHRA PUBLICATION)Chapter:STOICHIOMETRY Exercise:VERY SHORT Answer questions
Explore 13 Videos
- STATES OF MATTER GASES AND LIQUIDS Book:VIKRAM PUBLICATION ( ANDHRA PUBLICATION)Chapter:STATES OF MATTER GASES AND LIQUIDS Exercise:LONG ANSWER QUESTIONS
Explore 22 Videos
- THE P-BLOCK ELEMENTS GROUP-13Book:VIKRAM PUBLICATION ( ANDHRA PUBLICATION)Chapter:THE P-BLOCK ELEMENTS GROUP-13Exercise:Long Answer Questions
Explore 3 Videos
Similar Questions
Write the balanced ionic equation which represents the oxidation of iodine (I−) ion by per-manganate ion in basic medium to give iodine (I) and manganese dioxide (MnO2).
View SolutionHow many number of moles of glucose are present in 540 gms of glucose ?
View SolutionHow many number of moles of glucose are present in 540 gms of glucose ?
View SolutionWrite notes on Intermolecular forces
View SolutionHow are borax and boric acid prepared ? Explain the action of heat on them.
View SolutionA compound on analysis gave the following percentage composition by weight: hydrogen = 9.09, oxygen = 36.36 carbon = 54.55
Its VD is 44. Find the molecular formula of the compound.
View SolutionAn organic compound contains carbon, hydrogen and oxygen. Its elemental analysis gaveC,38.71% and H,9.67%. The empirical formula of the compound would be :
View SolutionAn organic compound contains carbon, hydrogen and oxygen. Its chemical analysis gave C, 38.71% and H, 9.67%. The empirical formula of compound would be
View SolutionChemical analysis a carbon compound gave following percentage composition by weight of the element present, carbon = 10.06%, hydrogen = 0.84%, chlorine = 89.10%. Calculate the empirical formula of the compound.
View SolutionA carbon compound on analysis gave the following percentage composition, carbon 14.5%, hydrogen 1.8%, chlorine 64.46%, oxygen 19.24%. Calculate the empirical formula of the compound.
View Solutionఒక కర్బన సమ్మేళనాన్ని విశ్లేషించగా కింది సంఘటన శాతాలను ఇచ్చింది. కార్బన్ 14.5%, హైడ్రోజన్ 1.8%, క్లోరిన్ 64.46%, ఆక్సిజన్ 19.24% అయితే సమ్మేళనం అనుభావిక ఫార్ములా కనుక్కోండి .
View SolutionChemical analysis of a carbon compound gave the following percentage composition by weight of the elements present, carbon = 10.06% , hydrogen = 0.84% , chlorine = 89.10% . Calculate the empirical formula of the compound.
View SolutionAn organic compound on analysis gave the following composition. Carbon=40%, Hydrogen=6.66% and oxygen= 53.34%. Calculate its molecular formula if its molecular mass is 90.
View SolutionAn organic compound contains carbon hydrogen and oxygen. It elemental analysis gave C, 38.71% and H, 9.67% and O, 51.62%. The empirical formula of the compound would be __________.
View SolutionAn organic compound on analysis gave the following composition: carbon = 40%, hydrogen = 6.66% and oxygen = 53.34%. Calculate its molecular formula if its molecular mass is 90.
View Solution
