The electronic configuration of some elements are given below. (These are not actual symbols). Analyse the configurations and answer the follwing questions:
X - 2,8,6
Y-2,8,8
Z-2,8,1
a) Which element is chemically stable?
b) What is the atomic number of the element Z?
c) What is the nature of CHEMICAL BONDING in the compound formed between the element Z with element X?Solution in Malayalam
a) Y
b) 11
c) .Z_2X.
Topper's Solved these Questions
Similar Questions
Complete the chemical equations for the folowing ionisation reactions:
KCl→K++….
HNO3→H++…..
Mg(OH)2→…...+2OH−
H2SO4→2H++…...
NH4Cl→NH+4+…..
CaSO4→…........+SO2−4
View SolutionAtomic number means the number of …......... in an atom
View SolutionQuestions given below are based on electronic configurations of the elements. The three elements X,Y and Z with the electronic configuration shown below all form hydrides:
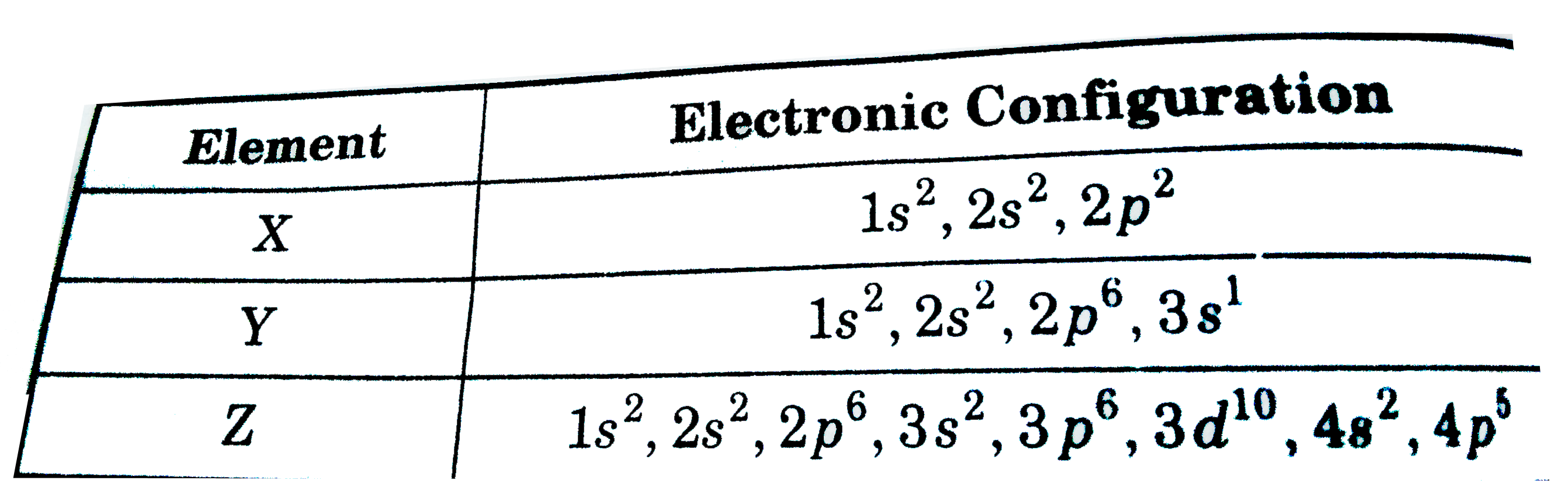
Which of the following exists as gas ?
View SolutionThe electronic configuration of an element Z is 2, 8, 8.
(a) What is the atomic number of the element ?
(b) State whether element Z is a metal or a non-metal
(c) What type of ion (if any) will be formed by an atom of element Z ? Why ?
(d) What is special about the outermost electron shell of the atom of this element ?
(e) Give the name and symbol of element Z
(f) Name the group of elements to which Z belongs.
View SolutionThe electronic configurations of two elements A and B are given below:
A 2,6
B 2,8,1
(a). What type of chemical bond is formed between the two atoms of A?
(b). What type of chemcial bond will be formed between the atoms of A and B?
View SolutionThe electronic configuration of an element Z is 2,8,6.How many electons does it require to have a stable configuration?
View SolutionAn element 'A' has atomic number 28. What is the electronic configuration of the elements below it in the periodic table? Predict their chemical properties.
View SolutionX, Y, Z तथा J तत्वों के इलेक्ट्रॉनिक विन्यास नीचे दिये गये है। इनमे से कौन-सा तत्व सबसे अधिक धात्विक है?
View SolutionThe electrons configuration of atom A is 2,8,6
What is the atomic number of element A ?
View SolutionThe electronic configuration of the elements X,Y, Z and J are given below . Which element has the highest metallic character
View Solutionఒక మూలక పరమాణువు A యొక్క ఎలక్ట్రాన్ విన్యాసం 2,8,6. అయితే ఆ మూలకం ఏ గ్రూపు కు చెం దుతోంది
View Solutionఒక మూలక పరమాణువు A యొక్క ఎలక్ట్రాన్ విన్యాసం 2,8,6. అ) A మూలకం యొక్క పరమాణు సంఖ్య ఎంత?\
View Solutionఒక మూలక పరమాణువు A యొక్క ఎలక్ట్రాన్ విన్యాసం 2,8,6. అ) A మూలకం యొక్క పరమాణు సంఖ్య ఎంత?\
View SolutionElement 'A' has Electronic confguration 2,7 'B' has configuration 2,8,6, 'C' has configuration 2,8,8 while 'D' has 2,8,7. Which element will show similar chemical properties?
View SolutionThe electronic configuration of three elements X, Y and Z are as follows: X to 2, 8 Y to 2,8,6 Z to 2,8,1 Identify the metal and non-metal.
View Solution
