A part of electrodes series is given. Observe the values and fill up the following based on datas given:
'(##VPS_HSS_CHE_ XII_C03_E03_004_Q01##)'
i Ai: Oxidation , Pb....
ii. From the given,electrodes, which are to be chosen inorder to construct'a galvanic cell which can provide the maximumi value of emf?
iii. What will be the emf value of that cell? Find out the anode, cathode and chemical reaction in the cell?
i. Al: Oxidation : Pb: reduction - Cu: Oxidation : Ag : reduction Zn: Oxidation : Ni: reduction ii. AlAl2:/Ag+Ag can provide the maximum value of emf. "iii. EMF=Ep−EL=+0.8−(−1.66)=2.46~V anode: Al, Cathode :Ag reaction: A+3Ag⋅Al21++3Ag
Topper's Solved these Questions
Similar Questions
Write the formulas for the following coordination compounds: i) Tetraamminediaquacobalt(III) chioride ii) Potassium tetracyanonickelate(II) iii) Tris(ethane-1,2-diamine)' chromium(III) chioride s iv) Ámminebromidochloridonitrito-N-platinațe(II) v) Dichloridóbis(ethane-l,2-diamine) platinum (iv) nitrate vi) Iron(III) hexacyanoferrate (II)
View SolutionAnalyses the table given below:
 Which of the ores mentioned in the above table can be concentrated by magnetic separation method? Justify your answer.
Which of the ores mentioned in the above table can be concentrated by magnetic separation method? Justify your answer.
View SolutionConsider the following relations for emf of a electrochemical cell
(i) emf of cell = (Oxidation potential of anode)-(Reduction potential of cathode)
(ii) emf of cell = (Oxidation potential of anode)+(Reduction potential of cathode)
(iii) emf of cell = (Reduction potential of anode)+(Reduction potential of cathode)
(iv) emf of cell = (Oxidation potential of anode)-(Oxidation potential of cathode)
Which of the above realtions are correct?
View SolutionConsider the following relations for
(i) EMF of the cell
=(Oxidation potential of anode)
-(Reduction potential of cathode)
(iii) EMF of the cell=(Reduction potential of anode) + (Reduction potential of cathode) (iv) EMF of the cell=(Oxidation potential of the anode)-(Oxidation potential of the cathode) which of the following above reactions are correct?
View SolutionThe standard oxidation potential of silver electrode is given as - 0.799 V . The standard reduction potential of copper electrode is given as 0.33 V. Find out the emf of the cell constituted by these two electrodes. Also write the half -cell reactions involved in the cell and given cell representation
View SolutionAssertion :EMF of the cell is the potential difference between the electrode potentials of the cathode and anode when no current is drawn through the cell.
Reason: Anode is kept on the right side and cathode on the left side while representing the galvanic cell.
View SolutionConsider the following relations for emf of a electrochemical cell
(i) emf of cell = (Oxidation potential of anode)-(Reduction potential of cathode)
(ii) emf of cell = (Oxidation potential of anode)+(Reduction potential of cathode)
(iii) emf of cell = (Reduction potential of anode)+(Reduction potential of cathode)
(iv) emf of cell = (Oxidation potential of anode)-(Oxidation potential of cathode)
Which of the above realtions are correct?
View SolutionConsider the following relations for emf of a electrochemical cell
(i) emf of cell = (Oxidation potential of anode)-(Reduction potential of cathode)
(ii) emf of cell = (Oxidation potential of anode)+(Reduction potential of cathode)
(iii) emf of cell = (Reduction potential of anode)+(Reduction potential of cathode)
(iv) emf of cell = (Oxidation potential of anode)-(Oxidation potential of cathode)
Which of the above realtions are correct?
View SolutionIn a galvanic cell, the electrode which acts as anode is
View SolutionElectrode Potential | Emf Of Galvanic Cell
View Solutionএকটি গ্যালভানিয় কোষ গঠন করার জন্য দুটি তড়িৎদ্দ্বার দেওয়া হল । এই দুটি তড়িৎদ্দ্বারের প্রমাণ বিজারণ বিভবের মান জানা থাকলে কোনটিকে অ্যানোড ও ক্যাথোড রূপে ব্যবহার করবে ?
View SolutionThe two half cell reactions of an electrochemical cell is given as
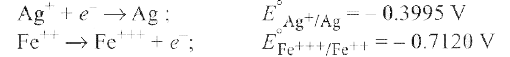
the value of cell EMF will be
View SolutionIn a galvanic cell cathode is positive electrode and anode is negative electrode.
View SolutionThe emf of a galvanic cell composed of two hydrogen electrodes is 272mV. What is the pH of the solution in which the anode is immersed if the cathode is in a solution with a pH of 3?
View SolutionConsider the following relations for emf of a electrochemical cell (i) emf of cell = (Oxidation potential of anode)-(Reduction potential of cathode) (ii) emf of cell = (Oxidation potential of anode)+(Reduction potential of cathode) (iii) emf of cell = (Reduction potential of anode)+(Reduction potential of cathode) (iv) emf of cell = (Oxidation potential of anode)-(Oxidation potential of cathode) Which of the above realtions are correct?
View Solution

