Which of the following form an alloy?
Ag+Pb
Fe+Hg
Pt+Hg
Fe+C
To determine which of the given options forms an alloy, we will analyze each pair of metals provided in the question.
Step 1: Analyze the first option - Silver (Ag) and Lead (Pb)
- When Silver (Ag) is mixed with Lead (Pb), they form an alloy known as Argentiferous lead.
- Conclusion: Yes, Ag and Pb form an alloy.
Step 2: Analyze the second option - Iron (Fe) and Mercury (Hg)
- Iron (Fe) does not form an alloy with Mercury (Hg) because Hg is a liquid and does not diffuse into the tightly packed lattice of iron.
- Conclusion: No, Fe and Hg do not form an alloy.
Step 3: Analyze the third option - Platinum (Pt) and Mercury (Hg)
- Similar to iron, Platinum (Pt) does not form an alloy with Mercury (Hg) for the same reason; Pt has a strong and compact lattice structure that does not allow Hg to diffuse into it.
- Conclusion: No, Pt and Hg do not form an alloy.
Step 4: Analyze the fourth option - Iron (Fe) and Carbon (C)
- When Iron (Fe) is mixed with Carbon (C), they form an alloy known as cementite, with the formula Fe3C.
- Conclusion: Yes, Fe and C form an alloy.
Final Conclusion:
- The pairs that form alloys are:
- Silver (Ag) and Lead (Pb) - Argentiferous lead
- Iron (Fe) and Carbon (C) - Cementite
- The pairs that do not form alloys are:
- Iron (Fe) and Mercury (Hg)
- Platinum (Pt) and Mercury (Hg)
Summary of Results:
- Alloys Formed: Ag + Pb, Fe + C
- No Alloys Formed: Fe + Hg, Pt + Hg
- When Silver (Ag) is mixed with Lead (Pb), they form an alloy known as Argentiferous lead.
- Conclusion: Yes, Ag and Pb form an alloy.
- Iron (Fe) does not form an alloy with Mercury (Hg) because Hg is a liquid and does not diffuse into the tightly packed lattice of iron.
- Conclusion: No, Fe and Hg do not form an alloy.
- Similar to iron, Platinum (Pt) does not form an alloy with Mercury (Hg) for the same reason; Pt has a strong and compact lattice structure that does not allow Hg to diffuse into it.
- Conclusion: No, Pt and Hg do not form an alloy.
- When Iron (Fe) is mixed with Carbon (C), they form an alloy known as cementite, with the formula Fe3C.
- Conclusion: Yes, Fe and C form an alloy.
- The pairs that form alloys are:
- Silver (Ag) and Lead (Pb) - Argentiferous lead
- Iron (Fe) and Carbon (C) - Cementite
- The pairs that do not form alloys are:
- Iron (Fe) and Mercury (Hg)
- Platinum (Pt) and Mercury (Hg)
- Alloys Formed: Ag + Pb, Fe + C
- No Alloys Formed: Fe + Hg, Pt + Hg
Topper's Solved these Questions
- D BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:D BLOCK ELEMENTSExercise:MISCELLANEOUS SOLVED PROBLEM (MSPs)
Explore 14 Videos
- D BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:D BLOCK ELEMENTSExercise:BOARD LEVEL EXERCISE
Explore 21 Videos
- D & F BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:D & F BLOCK ELEMENTSExercise:ORGANIC CHEMISTRY(Aldehydes , Ketones, Carboxylic acid)
Explore 15 Videos
- DPPBook:RESONANCE ENGLISHChapter:DPPExercise:QUESTIONS
Explore 368 Videos
Similar Questions
Knowledge Check
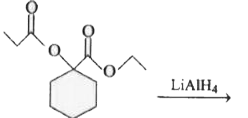
Which of the following will not form?A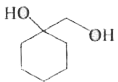 B
B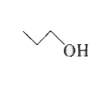 C
C D
D
Which of the following forms hydrate?
View SolutionWhich of the following form tetrahydridoborates
View SolutionWhich of the following is formed in nucleolus
View SolutionWhich of the following alloys contain (s) Cu and Zn?
View SolutionAn alloy is
View SolutionWhich of the following is not correct for alloys
View SolutionIn which of the following alloys Mg is not present ?
View Solution
