Which of the following option(s) is/are independent of both n and Z for H−like species?
Un= Potential energy of electron in nth orbit
KEn= Kinetic energy of electron in nth orbit
ln= Angular momentam of electoron in nth orbit
vn= Velcity of electron in nth orbit
fn= Frequency of electron in nth orbit
Tn= Time period of revolution of electron in nth orbit
rnUn
rn×KEn×ln
Un×Tnln
ln×fnv2n
The correct Answer is:C, D
To solve the question of which of the following options is/are independent of both n and Z for H−like species, we will analyze each option step by step.
1. Understanding the Variables:
- Un: Potential energy of electron in the nth orbit.
- KEn: Kinetic energy of electron in the nth orbit.
- Ln: Angular momentum of electron in the nth orbit.
- vn: Velocity of electron in the nth orbit.
- fn: Frequency of electron in the nth orbit.
- Tn: Time period of revolution of electron in the nth orbit.
2. Analyzing Each Option:
- Option A: \( U_n \)
The potential energy is given by:
\(
U_n = -\frac{2E_0 Z^2}{n^2}
\)
This expression clearly depends on both n and Z, so it is not independent.
- Option B: \( KE_n \)
The kinetic energy is given by:
\(
KE_n = \frac{E_0 Z^2}{n^2}
\)
This also depends on both n and Z, so it is not independent.
- Option C: \( L_n \)
The angular momentum is given by:
\(
L_n = \frac{nh}{2\pi}
\)
This expression only depends on n and is independent of Z, but since it depends on n, it is not independent of both.
- Option D: \( v_n \)
The velocity is given by:
\(
v_n = \frac{Z e^2}{h} \cdot \frac{1}{n}
\)
This expression depends on both n and Z, so it is not independent.
- Option E: \( f_n \)
The frequency is given by:
\(
f_n = \frac{E_0 Z^2}{h}
\)
This expression depends on Z, so it is not independent.
- Option F: \( T_n \)
The time period is given by:
\(
T_n = \frac{1}{f_n} = \frac{h}{E_0 Z^2}
\)
This expression also depends on Z, so it is not independent.
3. Finding Combinations:
- Option G: \( U_n \cdot T_n / L_n \)
We can analyze this combination:
\(
\frac{U_n \cdot T_n}{L_n} = \frac{-\frac{2E_0 Z^2}{n^2} \cdot \frac{h}{E_0 Z^2}}{\frac{nh}{2\pi}} = \frac{-4\pi}{n^3}
\)
This expression depends on n but not on Z.
- Option H: \( L_n \cdot f_n / v_n^2 \)
Analyzing this combination:
\(
\frac{L_n \cdot f_n}{v_n^2} = \frac{\frac{nh}{2\pi} \cdot \frac{E_0 Z^2}{h}}{\left(\frac{Z e^2}{h} \cdot \frac{1}{n}\right)^2}
\)
This simplifies to:
\(
\frac{nh E_0 Z^2}{2\pi} \cdot \frac{n^2 h^2}{Z^2 e^4} = \frac{n^3 E_0 h}{2\pi e^4}
\)
This expression depends on n but not on Z.
4. Conclusion:
After analyzing all options, we find that:
- The combinations \( U_n \cdot T_n / L_n \) and \( L_n \cdot f_n / v_n^2 \) are independent of Z but dependent on n.
Final Answer:
The options that are independent of both n and Z are:
- None of the options provided are completely independent of both n and Z.
- Un: Potential energy of electron in the nth orbit.
- KEn: Kinetic energy of electron in the nth orbit.
- Ln: Angular momentum of electron in the nth orbit.
- vn: Velocity of electron in the nth orbit.
- fn: Frequency of electron in the nth orbit.
- Tn: Time period of revolution of electron in the nth orbit.
- Option A: \( U_n \)
The potential energy is given by:
\(
U_n = -\frac{2E_0 Z^2}{n^2}
\)
This expression clearly depends on both n and Z, so it is not independent.
The kinetic energy is given by:
\(
KE_n = \frac{E_0 Z^2}{n^2}
\)
This also depends on both n and Z, so it is not independent.
The angular momentum is given by:
\(
L_n = \frac{nh}{2\pi}
\)
This expression only depends on n and is independent of Z, but since it depends on n, it is not independent of both.
The velocity is given by:
\(
v_n = \frac{Z e^2}{h} \cdot \frac{1}{n}
\)
This expression depends on both n and Z, so it is not independent.
The frequency is given by:
\(
f_n = \frac{E_0 Z^2}{h}
\)
This expression depends on Z, so it is not independent.
The time period is given by:
\(
T_n = \frac{1}{f_n} = \frac{h}{E_0 Z^2}
\)
This expression also depends on Z, so it is not independent.
- Option G: \( U_n \cdot T_n / L_n \)
We can analyze this combination:
\(
\frac{U_n \cdot T_n}{L_n} = \frac{-\frac{2E_0 Z^2}{n^2} \cdot \frac{h}{E_0 Z^2}}{\frac{nh}{2\pi}} = \frac{-4\pi}{n^3}
\)
This expression depends on n but not on Z.
Analyzing this combination:
\(
\frac{L_n \cdot f_n}{v_n^2} = \frac{\frac{nh}{2\pi} \cdot \frac{E_0 Z^2}{h}}{\left(\frac{Z e^2}{h} \cdot \frac{1}{n}\right)^2}
\)
This simplifies to:
\(
\frac{nh E_0 Z^2}{2\pi} \cdot \frac{n^2 h^2}{Z^2 e^4} = \frac{n^3 E_0 h}{2\pi e^4}
\)
This expression depends on n but not on Z.
After analyzing all options, we find that:
- The combinations \( U_n \cdot T_n / L_n \) and \( L_n \cdot f_n / v_n^2 \) are independent of Z but dependent on n.
The options that are independent of both n and Z are:
- None of the options provided are completely independent of both n and Z.
टॉपर्स ने हल किए ये सवाल
- TEST PAPERSBOOK - RESONANCE ENGLISHCHAPTER - TEST PAPERSEXERCISE - PART - III CHEMISTRY
46 Videos
- TEST PAPERSBOOK - RESONANCE ENGLISHCHAPTER - TEST PAPERSEXERCISE - PART - III CHEMISTRY
20 Videos
- TEST PAPERSBOOK - RESONANCE ENGLISHCHAPTER - TEST PAPERSEXERCISE - PART - III CHEMISTRY SEC - 1
12 Videos
- SURFACE CHEMISTRYBOOK - RESONANCE ENGLISHCHAPTER - SURFACE CHEMISTRYEXERCISE - Section - 5
1 Video
- TEST SERIESBOOK - RESONANCE ENGLISHCHAPTER - TEST SERIESEXERCISE - CHEMISTRY
50 Videos
समान प्रश्न
Which of the following compound contains maximum number of oxygen atoms ?
View Solution16 g of oxygen and 4 g of hydrogen are placed in 1.12 litre flask at 0∘C. Find the total pressure in atm of the gas mixture.
View Solution10dm3 of N2 gas and 10dm3 of gas X contain the same number of molecules at the same temperature, the gas (X) may be
View SolutionMatch list I with list II and select the correct answer :
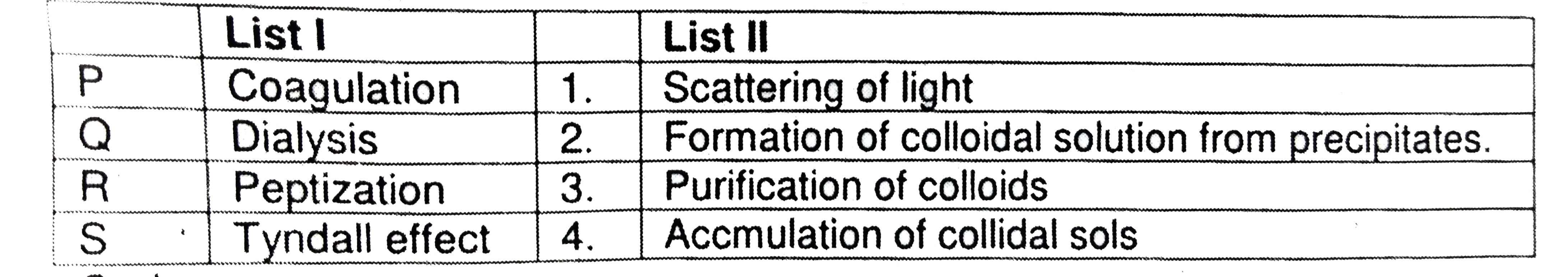
View Solutionwhich of the following plots will obtained for a conductometric titration of strong acid against a weak base?
View SolutionWhich of the following option(s) is/are independent of both n and Z for H−like species?
Un= Potential energy of electron in nth orbit
KEn= Kinetic energy of electron in nth orbit
ln= Angular momentam of electoron in nth orbit
vn= Velcity of electron in nth orbit
fn= Frequency of electron in nth orbit
Tn= Time period of revolution of electron in nth orbit
View SolutionIn the Bohr model of the atom (i) the radius of the n^(th) orbit is proportional to n^(2) (ii) the total energy of the electron in n^(th) orbit is inversely proportional to n (iii) the angular momentum of the electron in an orbit is an integral multiple of h//2 pi (iv) the magnitude of the P.E. of the electron in an orbit is gt its K.E.
View SolutionThe angular momentum of electron in nth orbit is given by
View SolutionIf in hydrogen atom, radius of nth Bohr orbit is , nr frequency of revolution of electron in nth orbit is fn choose the correct option
View SolutionThe energy of an electron in n^"th" orbit of hydrogen atom is
View SolutionThe angular momentum of an electron in nth orbit is given by
View SolutionThe difference between the radii of n^(th) and (n + 1)^(th) orbits of hydrogen atoms is equal to the radius of (n-1)^(th) orbit of hydrogen. The angular momentum of the electron in the n^(th) orbit is ___ (h is Planck's constant)
View Solution" Energy of the nth orbit "-
View SolutionKinetic energy of electron in n^("th") orbit in H-atom
View SolutionH-परमाणु के nth बोहर कक्षक में इलेक्ट्रॉन की ऊर्जा है
View SolutionEnergy of an electron in n^(th) Bohr orbit is given as
View Solution

