In the balanced chemical reaction
IOө3+alө+bHө→cH2O+dI2
a,b,c, and d, respectively, correspond to
a=5
c=3
d=5
b=6
The correct Answer is:A, B, D
To solve the balanced chemical reaction given by:
IO−3+Al+bH→cH2O+dI2
we need to balance the equation step by step.
Step 1: Identify the oxidation states
First, we need to identify the oxidation states of the elements involved in the reaction. In IO−3, iodine is in the +5 oxidation state. The aluminum (Al) is in the 0 oxidation state, and hydrogen (H) is also in the 0 oxidation state.
Step 2: Determine the products
The products of the reaction include water (H2O) and iodine (I2). In I2, iodine is in the 0 oxidation state.
Step 3: Write the half-reactions
Next, we can write the half-reactions for the oxidation and reduction processes.
1. Reduction half-reaction:
IO−3+6H++5e−→I2+3H2O
2. Oxidation half-reaction:
Al→Al3++3e−
Step 4: Balance the electrons
To balance the electrons transferred, we need to multiply the oxidation half-reaction by 5 to match the 5 electrons in the reduction half-reaction:
5(Al→Al3++3e−)→5Al→5Al3++15e−
Step 5: Combine the half-reactions
Now, we can combine the half-reactions:
IO−3+5Al+6H+→I2+3H2O+5Al3+
Step 6: Identify coefficients
Now we can identify the coefficients a,b,c, and d in the original equation:
- a (for Al) = 5
- b (for H) = 6
- c (for H2O) = 3
- d (for I2) = 3
Final Answer
Thus, the values are:
- a=5
- b=6
- c=3
- d=3
Summary
The balanced equation gives us the values:
- A=5
- B=6
- C=3
- D=3
First, we need to identify the oxidation states of the elements involved in the reaction. In IO−3, iodine is in the +5 oxidation state. The aluminum (Al) is in the 0 oxidation state, and hydrogen (H) is also in the 0 oxidation state.
The products of the reaction include water (H2O) and iodine (I2). In I2, iodine is in the 0 oxidation state.
Next, we can write the half-reactions for the oxidation and reduction processes.
IO−3+6H++5e−→I2+3H2O
Al→Al3++3e−
To balance the electrons transferred, we need to multiply the oxidation half-reaction by 5 to match the 5 electrons in the reduction half-reaction:
Now, we can combine the half-reactions:
Now we can identify the coefficients a,b,c, and d in the original equation:
- b (for H) = 6
- c (for H2O) = 3
- d (for I2) = 3
Thus, the values are:
- a=5
- b=6
- c=3
- d=3
The balanced equation gives us the values:
- A=5
- B=6
- C=3
- D=3
Topper's Solved these Questions
- TEST PAPERSBOOK - RESONANCE ENGLISHCHAPTER - TEST PAPERSEXERCISE - PART - III CHEMISTRY
20 Videos
- TEST PAPERSBOOK - RESONANCE ENGLISHCHAPTER - TEST PAPERSEXERCISE - PT-01
30 Videos
- TEST PAPERSBOOK - RESONANCE ENGLISHCHAPTER - TEST PAPERSEXERCISE - PART - III CHEMISTRY SEC - 2
18 Videos
- SURFACE CHEMISTRYBOOK - RESONANCE ENGLISHCHAPTER - SURFACE CHEMISTRYEXERCISE - Section - 5
1 Video
- TEST SERIESBOOK - RESONANCE ENGLISHCHAPTER - TEST SERIESEXERCISE - CHEMISTRY
50 Videos
Similar Questions
16 g of oxygen and 4 g of hydrogen are placed in 1.12 litre flask at 0∘C. Find the total pressure in atm of the gas mixture.
View SolutionWhich of the following statement is not correct about the characteristics of cathode rays?
View SolutionWhat is power (in watt) of a light source emitting 3.125×1018 photons per second, while the wavelength of light is 620nm ? (use hc=12400eVÅ)
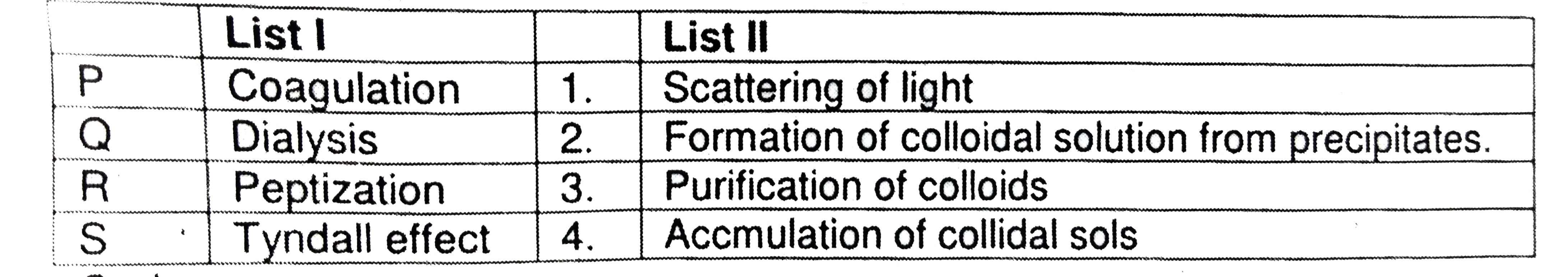
View SolutionMatch list I with list II and select the correct answer :
View Solutionwhich of the following plots will obtained for a conductometric titration of strong acid against a weak base?
View SolutionBalance the following reaction by oxidation number method:
CrO2−4+Iө→Cr3++IOө3 ( in alkaline or basic medium)
View SolutionWrite balanced net ionic equations for the following reactions in basic solution: (a) H_(2)O_(2)(aq)+ClO_(4)^(Ө)(aq) rarr ClO_(2)^(Ө)(aq)+O_(2)(g) (b) ( c) Cu(OH)_(2)(s) + N_(2)H_(4)(aq) rarr Cu(s)+N_(2)(g) (d) MnO_(4)^(Ө) + IO_(3)^(Ө)(aq)rarr MnO_(2)(s) + IO_(4)^(Ө)(aq)
View SolutionBalanced the following equations: (a) H_(2)O_(2)+H^(o+)+Fe^(2+)rarr H_(2)O+Fe^(3+) (b) Ibr+BrO_(3)^(Ө)+H_(2)O rarrIO_(3)^(Ө)+Br^(Ө)+H^(o+) ( c) I^(Ө)+IO_(3)^(Ө) + H^(o+)rarrH_(2)O+I_(2) (d) underset("superoxide ion")(O_(2)^(Ө)+H_(2)O rarr overset(Ө)(OH)+O_(2)) (e) HS^(Ө)+HSO_(3)^(Ө) rarr S_(2)O_(3)^(2-)+H_(2)O
View SolutionIn the reaction 3Br_(2)+6CO_(3)^(2-)+3H_(2)Orarr5Br^(ө)+BrO_(3)^(ө)+6HCO_(3)^(ө)
View SolutionIn the balanced chemical reaction IO_(3)^(ө)+aI^(ө)+bH^(ө)rarrcH_(2)O+dI_(2) a, b,c , and d , respectively, correspond to
View SolutionIn the balanced chemical reaction IO_(3)^(-)+AI^(-)rarrCH_(2)O+dI_(2) a,b,c and d respectively correspond to
View SolutionIn the balanced chemical reaction IO_(3)^(-)+AI^(-)rarrCH_(2)O+dI_(2) a,b,c and d respectively correspond to
View SolutionIn the balanced chemical reaction, IO_(3)^(-) + aI^(-) + bH^(+) rarr cI_(2)+ dH_(2)O a,b,d and d respectively correspond to "_____________________"
View SolutionIn the reaction , IO_(3)^(-) + aI^(-) + bH^(+) to cH_(2)O + dI_(2) -
View SolutionCompare the stability of the following (a) overset(Ө)CH_(3) " " (b) overset(Ө)NH_(2)" " (c) overset(Ө)OH" "(d)overset(Ө)F
View SolutionIn the balance chemical equation :
IO−3aI−+bH+→cH2O+dI2 a,b,c and d respectively correspond to
View Solution

