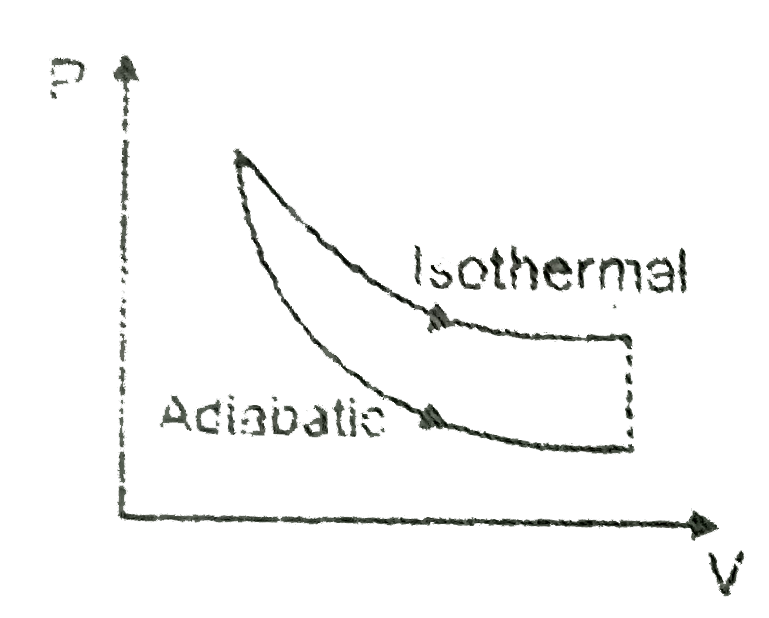
Let (ΔWa)and(ΔWb) be the work done by the system A and B respectively in the previous question.
ΔW1>ΔW2
ΔW1=ΔW2
ΔW1<ΔW2
The relation between W1 and W2 cannot be deduced.
The correct Answer is:A
As W.D. by gas in isothermal is more as compare to adiabatic process
∴ΔW2<ΔW1
Topper's Solved these Questions
- KTG & THERMODYNAMICSBOOK - RESONANCE ENGLISHCHAPTER - KTG & THERMODYNAMICSEXERCISE - SECTION (B)
3 Videos
- KTG & THERMODYNAMICSBOOK - RESONANCE ENGLISHCHAPTER - KTG & THERMODYNAMICSEXERCISE - SECTION (C)
3 Videos
- KTG & THERMODYNAMICSBOOK - RESONANCE ENGLISHCHAPTER - KTG & THERMODYNAMICSEXERCISE - SECTION (A)
3 Videos
- KINETIC THEORY OF GASES AND THERMODYNAMICSBOOK - RESONANCE ENGLISHCHAPTER - KINETIC THEORY OF GASES AND THERMODYNAMICSEXERCISE - Exercise
64 Videos
- MAGNETIC FIELD AND FORCESBOOK - RESONANCE ENGLISHCHAPTER - MAGNETIC FIELD AND FORCESEXERCISE - Exercise
64 Videos
Similar Questions
What is the total internal energy possessed by molecules of an ideal gas?
View SolutionState and explain first law of thermodynamics. Discuss the application of first law of thermodynamics to (i) isothermal process, (ii) adiabatic process.
View SolutionWhen a gas expands at constant temperature, why does the pressure decrease? Explanin it on the basis of kinetic theory of gases.
View SolutionThe ratio of r.m.s speed to the r.m.s angular speed of a diatomic gas at certain temperature is (assume m =mass of one molecule, M=molecular mass, I=moment of inertia of the molecules )
View SolutionOne tesla is equal to
View SolutionLet (ΔWa)and(ΔWb) be the work done by the system A and B respectively in the previous question.
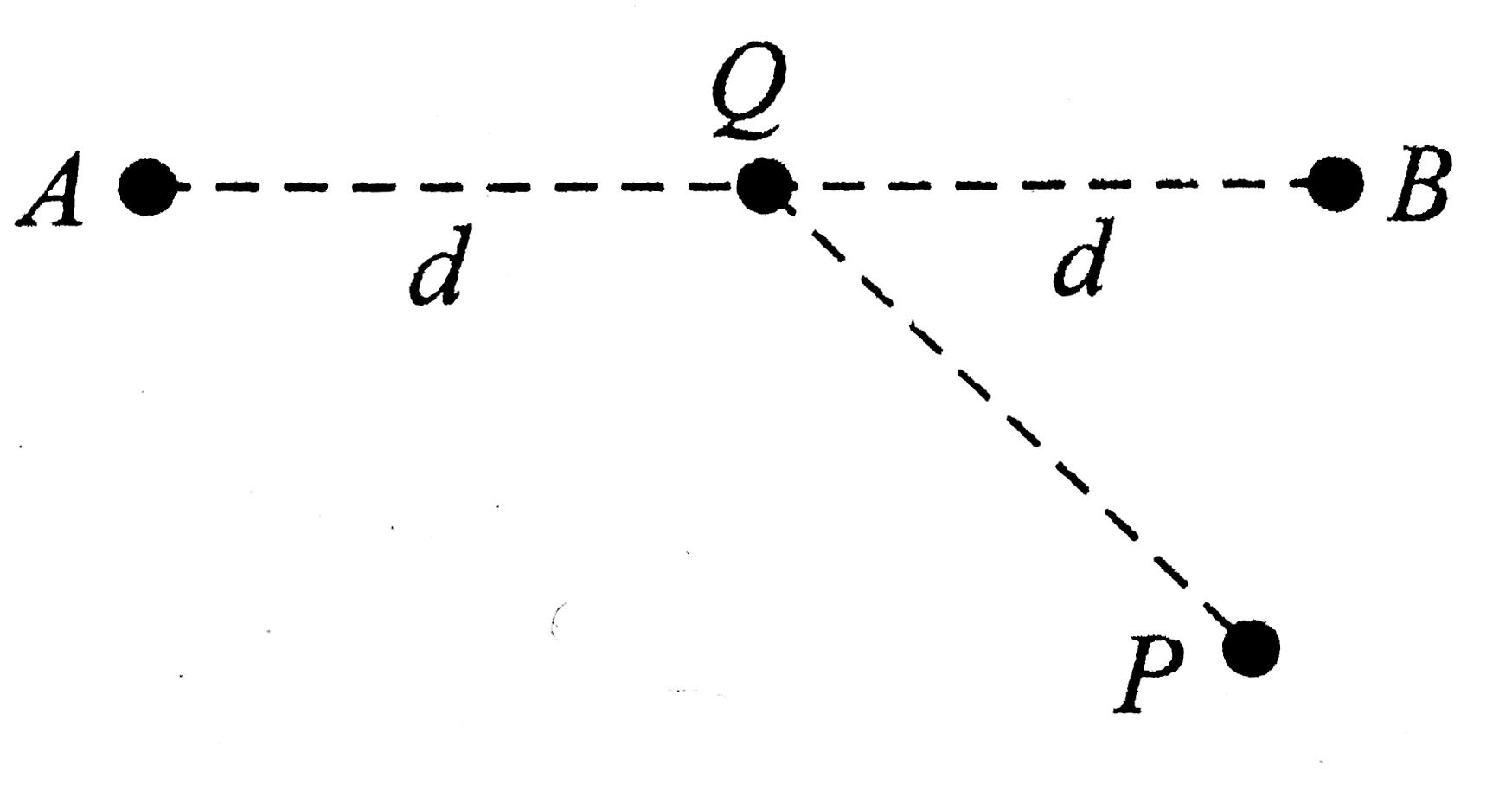
View SolutionConsider the situation of figure . The work done in taking a point charge form P to A isWA from P to B is WB from P to C is WC.

View SolutionThe work done in taking a unit positive charge from P to A is WA and P to B is WB. Then
 .
.
View SolutionIn given figure, let ΔW and ΔW2 be the work done by the gas in process A and B respectively then (given change in volume is same in both process)
View SolutionIf ΔU and ΔW represent the increase in internal energy and work done by the system resectively in a thermodynamical process, which of the following is true?
View SolutionIf ΔU and ΔW represent the increase in internal energy and work done by the system respectively in a thermodynamical process, which of the following is true ?
View SolutionIn given figure, let ΔW and ΔW2 be the work done by the gas in process A and B respectively then (given change in volume is same in both process)
View SolutionConsider the situation of figure . The work done in taking a point charge form P to A isWA from P to B is WB from P to C is WC.

View SolutionIf ΔUandΔW represent the increase in internal energy and work done by the system respectively in a thermodynamical process, which of the following is true?
View SolutionIf ΔUandΔW represent the increase in internal energy and work done by the system respectively in a thermodynamical process, which of the following is true?
View SolutionTwo springs A and B are identical but A is harder than B (kA>kB). Let Wand Wg represent the work done when the springs are stretched through the same distance and W.AandW.B are the work done when these are stretched by equal forces, then which of the following is true?
View Solution

