The following sets of values for Cv and Cp of an ideal gas have been reported by different students. The units are cal mole−1K−1. Which of these sets is most reliable?
Cv=3,Cp=5
Cv=4,Cp=6
Cv=3,Cp=2
Cv=3,Cp=4.2
The correct Answer is:A, B
To determine which set of values for Cp and Cv of an ideal gas is most reliable, we can use the relationship between Cp, Cv, and the universal gas constant R. The relationship is given by:
Cp−Cv=R
1. Identify the value of R:
The universal gas constant R is given as 8.314Jmol−1K−1. We need to convert this value into calories since the given values for Cp and Cv are in calories.
2. Convert R to calories:
We know that 1cal=4.186J. Therefore, to convert R from joules to calories, we divide by 4.186:
R=8.314Jmol−1K−14.186J/cal≈1.98calmol−1K−1≈2calmol−1K−1
3. Apply the relationship Cp−Cv=R:
Now that we have R≈2calmol−1K−1, we can check each set of values for Cp and Cv to see if they satisfy the equation Cp−Cv=2.
4. Evaluate each set:
- Set 1: Cp=5, Cv=3
Cp−Cv=5−3=2(Valid)
- Set 2: Cp=6, Cv=4
Cp−Cv=6−4=2(Valid)
- Set 3: Cp=2, Cv=3
Cp−Cv=2−3=−1(Invalid)
- Set 4: Cp=4.2, Cv=3
Cp−Cv=4.2−3=1.2(Invalid)
5. Conclusion:
The most reliable sets are Set 1 and Set 2, as they both satisfy the equation Cp−Cv=2.
The universal gas constant R is given as 8.314Jmol−1K−1. We need to convert this value into calories since the given values for Cp and Cv are in calories.
We know that 1cal=4.186J. Therefore, to convert R from joules to calories, we divide by 4.186:
Now that we have R≈2calmol−1K−1, we can check each set of values for Cp and Cv to see if they satisfy the equation Cp−Cv=2.
- Set 1: Cp=5, Cv=3
Cp−Cv=5−3=2(Valid)
- Set 2: Cp=6, Cv=4
Cp−Cv=6−4=2(Valid)
- Set 3: Cp=2, Cv=3
Cp−Cv=2−3=−1(Invalid)
- Set 4: Cp=4.2, Cv=3
Cp−Cv=4.2−3=1.2(Invalid)
The most reliable sets are Set 1 and Set 2, as they both satisfy the equation Cp−Cv=2.
টপার এই প্রশ্নগুলো সমাধান করেছে
- KTG & THERMODYNAMICSBook:RESONANCE ENGLISHChapter:KTG & THERMODYNAMICSExercise:PART -IV
Explore 9 Videos
- KTG & THERMODYNAMICSBook:RESONANCE ENGLISHChapter:KTG & THERMODYNAMICSExercise:Exercise-3
Explore 1 Video
- KTG & THERMODYNAMICSBook:RESONANCE ENGLISHChapter:KTG & THERMODYNAMICSExercise:PART -I
Explore 15 Videos
- KINETIC THEORY OF GASES AND THERMODYNAMICSBook:RESONANCE ENGLISHChapter:KINETIC THEORY OF GASES AND THERMODYNAMICSExercise:Exercise
Explore 64 Videos
- MAGNETIC FIELD AND FORCESBook:RESONANCE ENGLISHChapter:MAGNETIC FIELD AND FORCESExercise:Exercise
Explore 64 Videos
সম্পর্কিত প্রশ্ন
Comprehension-1
Two closed identical conducting containers are found in the laboratory of an old scientist. For the vertification of the gas some experiments are performed on the two boxes and the results are noted.
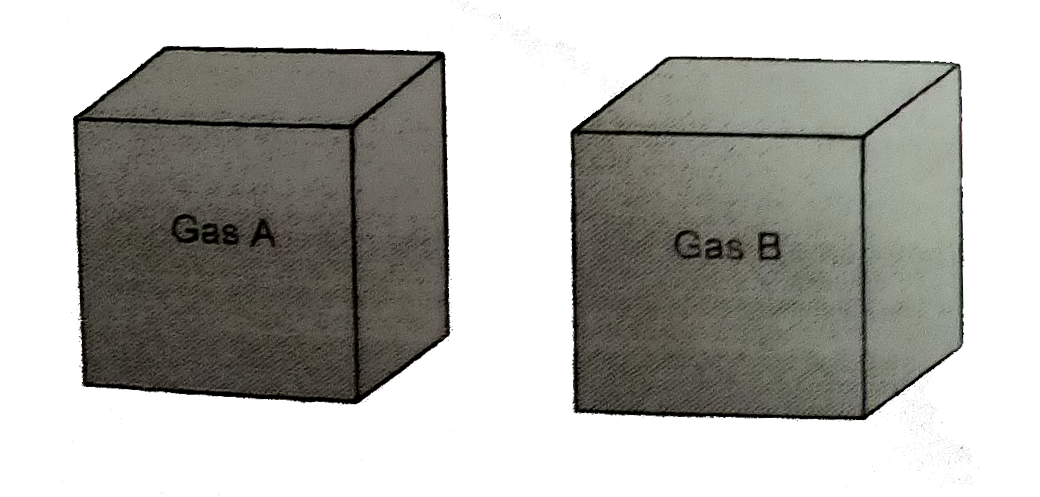
Experimenet 1.
When the two containers are weighed WA=225tg,WB=160g and mass of evacuated container WC=100g.
Experiment 2.
When the two containers are given same amount of heat same temperature rise is recorded. The pressure changes found are
ΔPA=2.5atm,ΔpB=1.5atm.
Required data for unknown gas:
∣∣ ∣ ∣ ∣ ∣∣Monomolar massHeNeArKrXeRd4g20g40g84g131g222gDiamolarmassH2F2N2O2CI22g19g28g32g71g∣∣ ∣ ∣ ∣ ∣∣
Total number of molecules in 'A' (here NA= Avagadro number)
View SolutionAn ideal gas is filled in a closed rigid and thermally insulated container. A coil of 100Ω resistor carrying current 1 A for 5 minutes supplies heat to the gas. The change in internal energy of the gas is
View SolutionThe given curve represents the variation of temperatue as a function of volume for one mole of an ideal gas. Which of the following curves best represents the variation of pressure as a function of volume ?
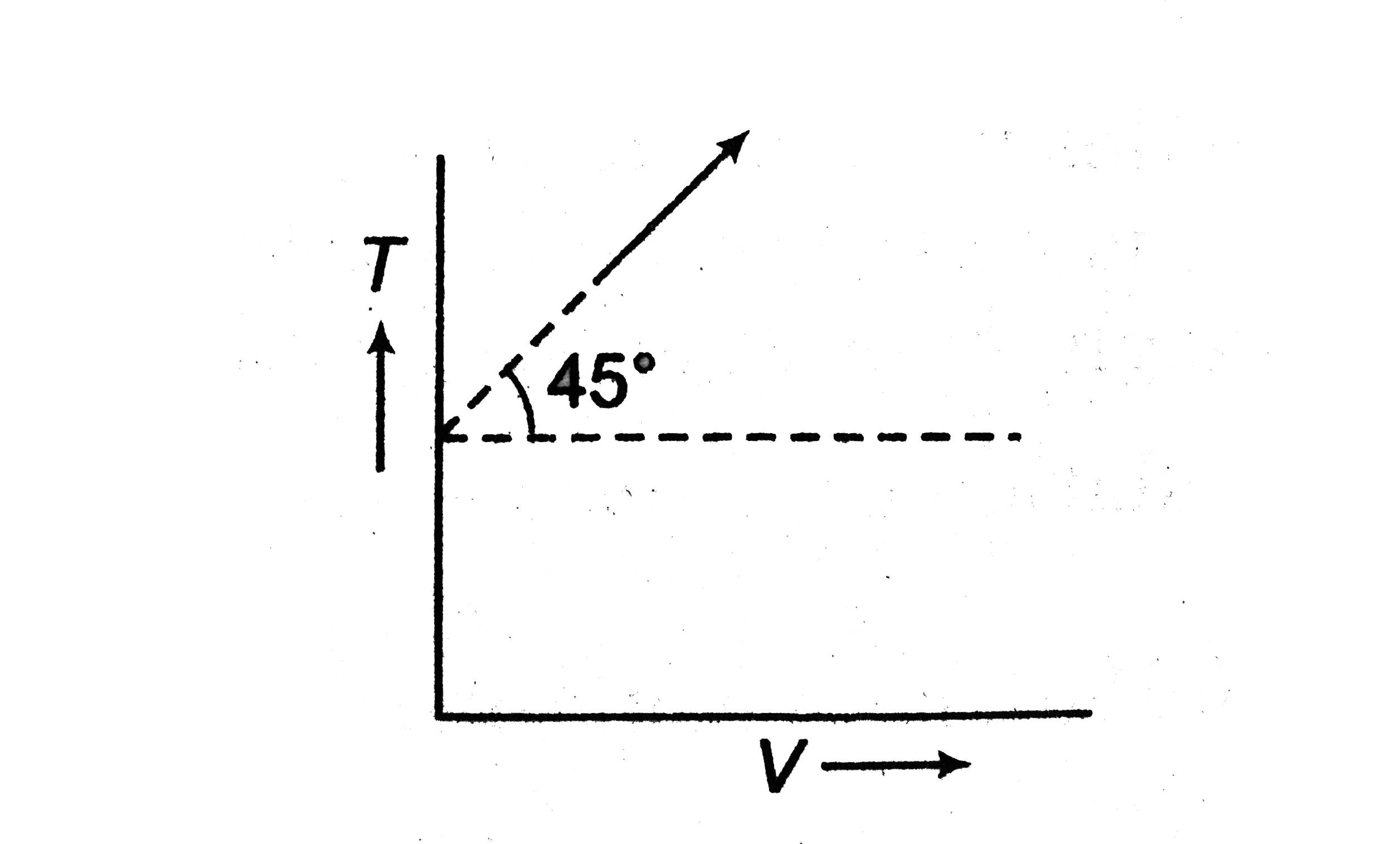 .
.
View SolutionThe ratio of r.m.s speed to the r.m.s angular speed of a diatomic gas at certain temperature is (assume m =mass of one molecule, M=molecular mass, I=moment of inertia of the molecules )
View SolutionOne tesla is equal to
View SolutionThe following sets of values for C_(v) and C_(p) of an ideal gas have been reported by different students. The units are cal "mole"^(-1)K^(-1) . Which of these sets is most reliable?
View SolutionOne mole of an ideal diatomic gas (Cv=5calK−1mole−1) chagre its state from state 1 (27∘C1L) to state 2 (127∘C,10L) . For this process, which of the following is (are) correct? (Given:R=2calK−1mole−1)
View SolutionThe difference between Cp and Cv can be derived using the empirical relation H = U + pV. Calculate the difference between Cp and Cv for 10 moles of an ideal gas.
View SolutionThe following sets of volume of Cv and Cp of an ideal gas have been reported by different students. The units are cal mole−1K_1. Which of these sets is most reliable ?
View SolutionThe difference between (C_(p)) and C_(v) can be derived usingthe empirical relation H = U + pV . Calculate the difference between C_(p) and C_(v) for 10 moles of an ideal gas.
View Solutionतीन विधार्थियों ने किसी गैस के लिए Cp तथा Cv के मानों के निम्नलखित सेट प्राप्त किये । इनमे कौन-सा सेट सबसे अधिक विश्वनीय है? सभी मान कैलोरी/मोल-K में है।
(i) Cp=5.0,Cv=3.1
(ii) Cp=6.0,Cv=4.0
(iii) Cp=4.2,Cv=3.0
View Solutionआदर्श गैस के लिए मेयर के सूत्र Cp−Cv=R की स्थापना कीजिए। प्रतीकों के सामान्य अर्थ है।
View SolutionFor an ideal gas C_(V,m)=12.47J*mol^(-1)*K^(-1) . What will be the value of C_(P,m) ?
View Solutionकिसी आदर्श गैस के लिए Cp−Cv का मान ........... होता है।
View Solutionएक आदर्श गैस के लिए Cp−CV का मान……….. होता है।
View SolutionThe molar specific heats C_(p) and C_(v) of an ideal gas are expressed in the unit of cal cdot mol^(-1) cdot^(@)C^(-1) . But R is expressed in J cdot mol^(-1) cdot^(@)C^(-1) . Write down the relation between C_(p) and C_(v) .
View Solution
