Figure shows Kα& Kβ X-rays along with continuous X-ray. Find the energy of Lα X-ray. (Use hc = 12420 evÅ)

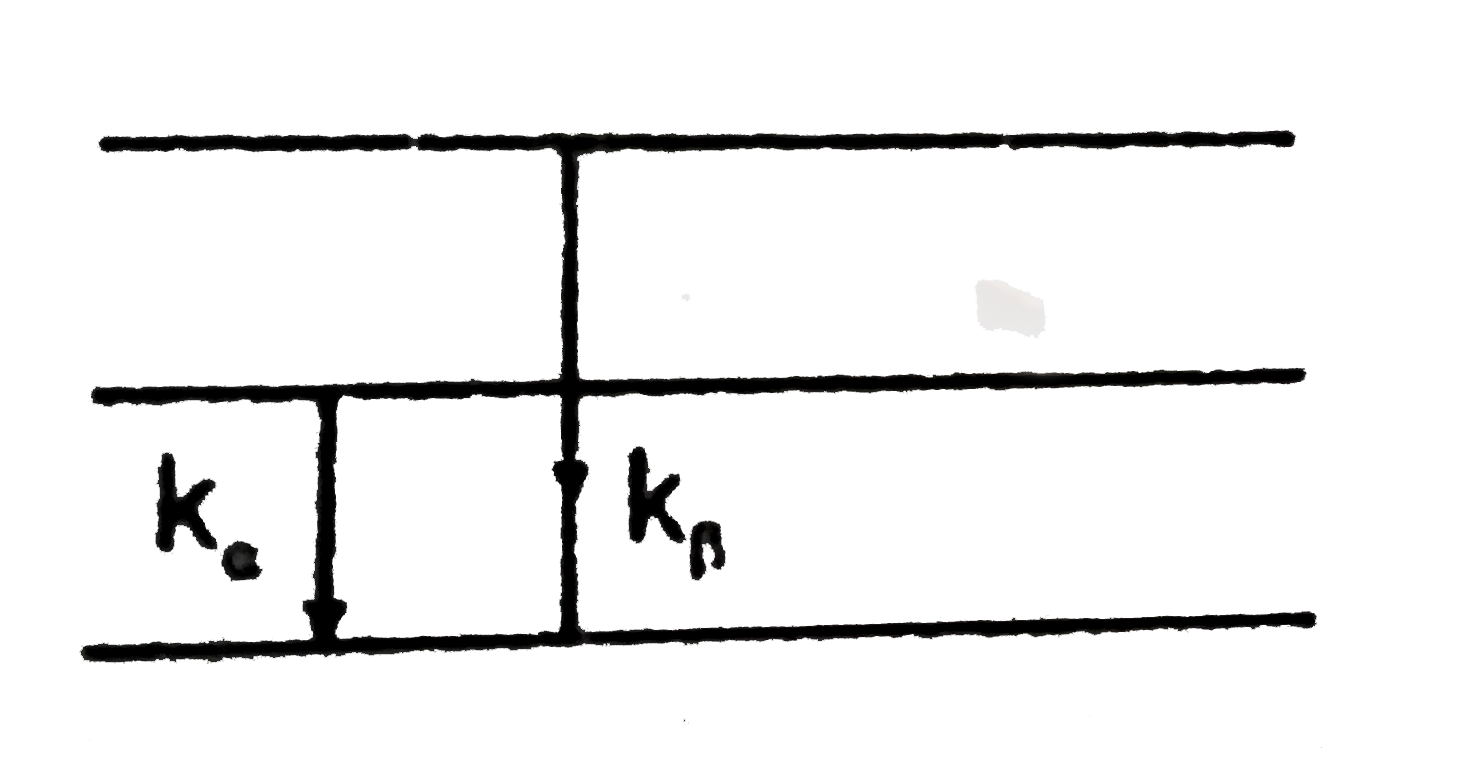
The correct Answer is:6.21KeV
=EKβ−EKα=12420(11−12)
=124202=6110Ev=6.21KeV.
Topper's Solved these Questions
- DAILY PRACTICE PROBLEMBook:RESONANCE ENGLISHChapter:DAILY PRACTICE PROBLEMExercise:DPP No.54
Explore 9 Videos
- DAILY PRACTICE PROBLEMBook:RESONANCE ENGLISHChapter:DAILY PRACTICE PROBLEMExercise:DPP No.55
Explore 8 Videos
- DAILY PRACTICE PROBLEMBook:RESONANCE ENGLISHChapter:DAILY PRACTICE PROBLEMExercise:DPP No.52
Explore 8 Videos
- CURRENT ELECTRICITYBook:RESONANCE ENGLISHChapter:CURRENT ELECTRICITYExercise:High Level Problems (HIP)
Explore 19 Videos
- ELECTRO MAGNETIC WAVESBook:RESONANCE ENGLISHChapter:ELECTRO MAGNETIC WAVESExercise:Exercise 3
Explore 27 Videos
Similar Questions
can X-rays be used for photoelectric effect?
View SolutionProduction of continuous X-rays is caused by
View Solution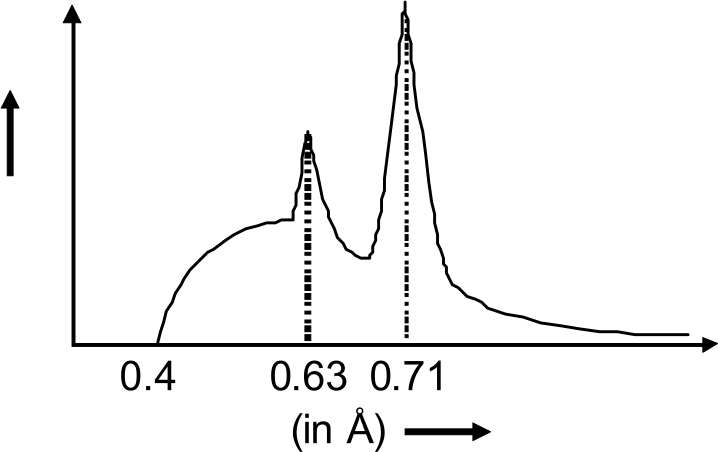
Figure shows intensity versus wavelength graph of X-rays coming from coolidge-tube with molybdenum as target element :
The two peaks shown in graph correspond to Kα and Kβ X-rays
Wavelength of Lα X-rays from Coolidge tube will be (approximately)
View SolutionThe Kα and Kβ X-rays of molybdenum have wavelengths 0⋅71A and0⋅63A respectively. Find the wavelength of Lα X-ray of molybdenum.
View SolutionFind the cut off walelength for the continuous X - rays coming
form an X-ray tube operating at 40 kv.
View SolutionFind the cut off wavelength for the continuous X - rays coming from an X-ray tube operating at 40 kV.
View SolutionAn element of atomic number 9 emits Kα X-ray of wavelength λ. Find the atomic number of the element which emits Kα X-ray of wavelength 4λ.
View SolutionFind the cutoff wavelength for the continuous X-rays coming form an X-ray tube operating at 30 kV.
View SolutionState any one difference between characteristic X-rays and continuous X-rays.
View SolutionIn figure find which is KaandLα'
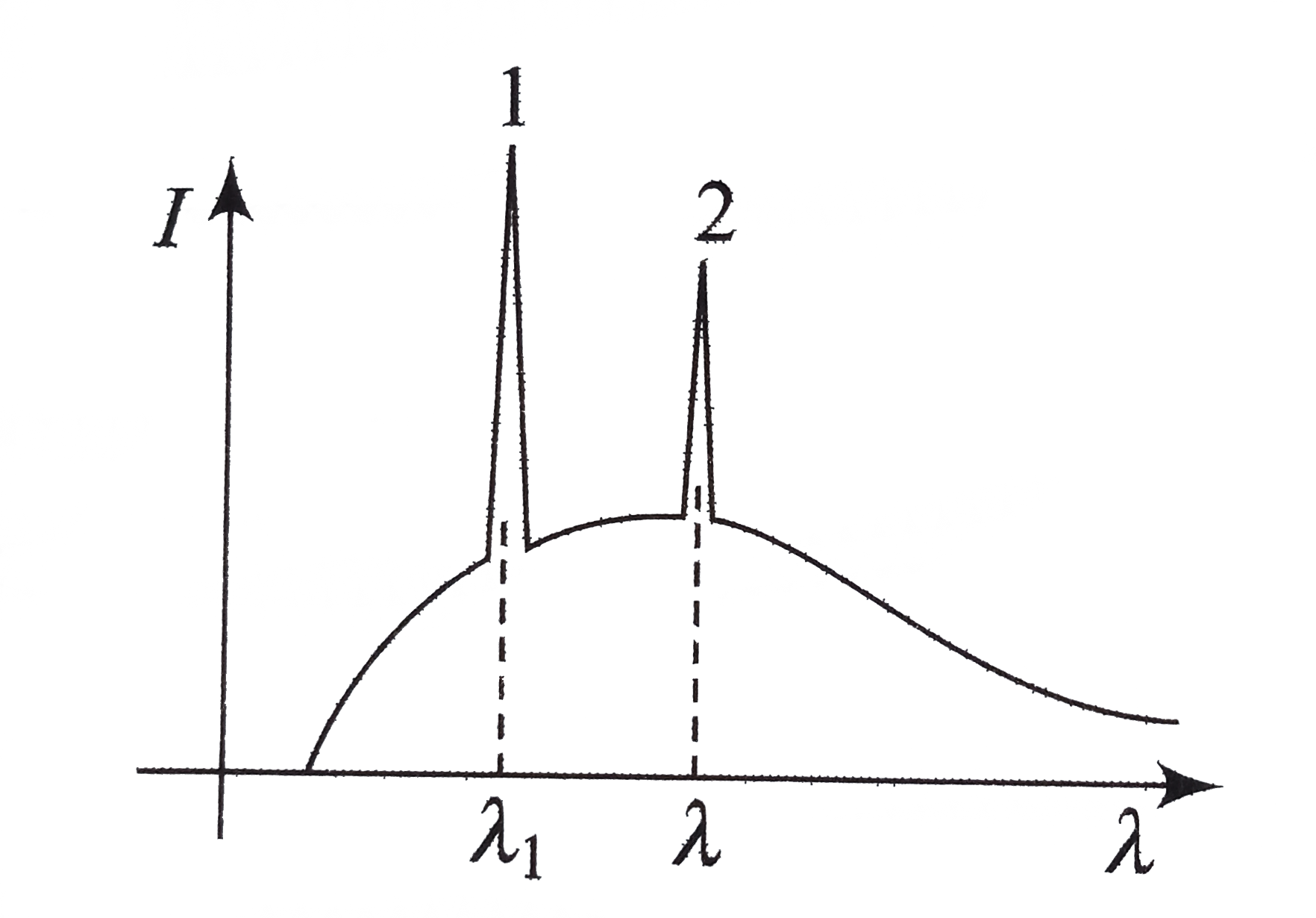
View Solution
