The binding energies of two nuclei Pn and Q2n and x and y joules. If 2x>y then the energy released in the reaction Pn+Pn→Q2n, will be
2x+y
2x-y
−(2x−y)
x+y
The correct Answer is:B
To solve the problem, we need to determine the energy released in the nuclear reaction Pn+Pn→Q2n given the binding energies of the nuclei involved.
1. Identify the Binding Energies:
- The binding energy of nucleus Pn is given as x joules.
- The binding energy of nucleus Q2n is given as y joules.
2. Calculate the Total Binding Energy of Reactants:
- Since we have two nuclei Pn in the reaction, the total binding energy of the reactants is:
Total Binding Energy of Reactants=x+x=2x
3. Calculate the Total Binding Energy of Products:
- There is one nucleus Q2n in the products, so the total binding energy of the products is:
Total Binding Energy of Products=y
4. Determine the Energy Released in the Reaction:
- The energy released in the reaction can be calculated using the formula:
Energy Released=Binding Energy of Products−Binding Energy of Reactants
- Substituting the values we calculated:
Energy Released=y−2x
5. Rearranging the Expression:
- To express this in a more standard form:
Energy Released=−(2x−y)
6. Final Result:
- Therefore, the energy released in the reaction Pn+Pn→Q2n is:
Energy Released=−(2x−y)
Conclusion:
The energy released in the reaction is −(2x−y).
- The binding energy of nucleus Pn is given as x joules.
- The binding energy of nucleus Q2n is given as y joules.
- Since we have two nuclei Pn in the reaction, the total binding energy of the reactants is:
Total Binding Energy of Reactants=x+x=2x
- There is one nucleus Q2n in the products, so the total binding energy of the products is:
Total Binding Energy of Products=y
- The energy released in the reaction can be calculated using the formula:
Energy Released=Binding Energy of Products−Binding Energy of Reactants
- Substituting the values we calculated:
Energy Released=y−2x
- To express this in a more standard form:
Energy Released=−(2x−y)
- Therefore, the energy released in the reaction Pn+Pn→Q2n is:
Energy Released=−(2x−y)
The energy released in the reaction is −(2x−y).
Topper's Solved these Questions
Similar Questions
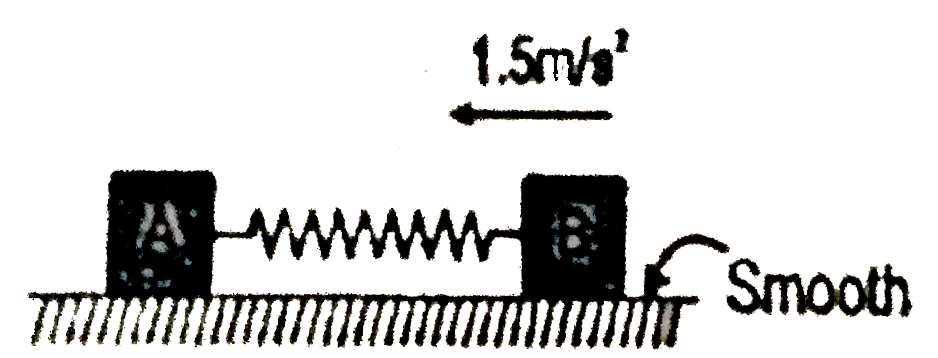
Two blocks A and With mass 4 kg and 6 kg respectively are connected by a stretched spiring of negligible mass as in figure. When the two blocks are released simultaneously the initial acceleration of B is 1.5m/s2 westward. The acceleration of A is :
View SolutionA velocity-time graph of two vehicals A & B starting from rest at the same time is given in the figure. The statements which is correct from graph.
View SolutionIn the expansion of (x+a)n if the sum of odd terms is P and the sum of even terms is Q, then (a)P2−Q2=(x2−a2)n (b)4PQ=(x+a)2n−(x−a)2n (c)2(P2+Q2)=(x+a)2n+(x−a)2n (d)none of these
View SolutionLet the co-efficients of x^(n) In (1+x)^(2n) and (1+x)^(2n-1) be P& Qrespectively,then ((P+Q)/(Q))^(5)=
View SolutionLet w is non-real root of x^3 =1 (i) If P = w^n, (n in N) and Q = (.^(2n)C_0 + .^(2n)C_3 +.....)+( .^(2n)C_1 + .^(2n) C_4+.....)w + (.^(2n)C_2 + .^(2n)C_2+.^(2n)C_5+....)w^2 then find P/Q
View SolutionThe binding energies of two nuclei Pn and Q2n and x and y joules. If 2x>y then the energy released in the reaction Pn+Pn→Q2n, will be
View Solutionif f(x) =∣∣ ∣ ∣∣mxmx−pmx−pnn+pn−pmx+2nmx+2n+pmx+2n−p∣∣ ∣ ∣∣ then
y=f(x) represents
View Solution(A)नाभिकों Xn तथा Y2n की बंधन ऊर्जायें क्रमशः P तथा Q जूल प्रति न्यूक्लिऑन हैं। यदि 2P<Q हो तो नाभिकीय अभिक्रिया Xn+Xn→Y2n में उत्सर्जित ऊर्जा ज्ञात कीजिए।
(B) एक नाभिक Xn की बंधन ऊॅर्जा P तथा दूसरे नाभिक Y2n की बंधन ऊर्जा Q है। n तथा 2n नाभिकों की द्रव्यमान संख्यायें हैं। यदि 2P>Q हो तो अभिक्रिया Xn+Xn→Y2n में ऊर्जा मुक्त होगी अथवा अवशोषित?
View Solutionनाभिको Xn तथा Y2n की बंधन -उर्जाये क्रमश: P तथा Q जूल प्रति न्यूक्लिऑन है! यदि 2P<Q हो, तो नाभिकीय अभिक्रिया Xn+Xn→Y2n में उतसर्जित ऊर्जा ज्ञात कीजिए!
View SolutionIf the greatest divisor 30⋅52n+4⋅23n is p,∀n∈N and that of 22n+1−6n−2 is q,∀n∈N, then p+q=
View Solutionदो नाभिको Pn व Q2n की बंधन ऊर्जा क्रमशः x जूल व y जूल है। यदि 2x>y, तो क्रिया Pn+Pn=Q2n में मुक्त ऊर्जा होगी
View SolutionIn the expansion of (x+a)n if the sum of odd terms is P and the sum of even terms is Q, then (a)P2−Q2=(x2−a2)n (b)4PQ=(x+a)2n−(x−a)2n (c)2(P2+Q2)=(x+a)2n+(x−a)2n (d)none of these
View SolutionIf P(n) :2n<n!,n∈N , then P(n) is true for all n≥…………...
View SolutionIf n lt p lt 2n and p is prime and N = .^(2n)C_n , then
View SolutionLet the co-efficients of xn In (1+x)2nand(1+x)2n−1 be P & Qrespectively, then (P+QQ)5=
View Solution
