A U238 sample of mass 1.0 g emits alpha particles at the rate 1.24×104 partcicles per second.
(NA=6.023×1023)
The half-life of this nuclide is 4.5×109 Years
The half life of this nuclide is 9×109 years
The activity of the prepared sample is 1.24×104 particles/sec
The activity of the prepared sample is 1.24×104 particles/sec
The correct Answer is:A, D
∣∣∣dNdt∣∣∣=λN=ln2T1/2×1×6.02×1023238
⇒T12=ln2×6.023×1023238×1.24×104=4.5×109Yrs.
The activity = number of disintergration per second =1.24×104dps
Topper's Solved these Questions
- NUCLEAR PHYSICSBook:RESONANCE ENGLISHChapter:NUCLEAR PHYSICSExercise:Exercise-2 Part-4 Comprehension
Explore 6 Videos
- NUCLEAR PHYSICSBook:RESONANCE ENGLISHChapter:NUCLEAR PHYSICSExercise:Exercise 3 Part -1 JEE (Advanced)
Explore 19 Videos
- NUCLEAR PHYSICSBook:RESONANCE ENGLISHChapter:NUCLEAR PHYSICSExercise:Exercise-2 Part -2 Single and double value interger type
Explore 15 Videos
- GRAVITATIONBook:RESONANCE ENGLISHChapter:GRAVITATIONExercise:HIGH LEVEL PROBLEMS
Explore 16 Videos
- REVISION DPPBook:RESONANCE ENGLISHChapter:REVISION DPPExercise:All Questions
Explore 463 Videos
Similar Questions
The radionuclide .56Mn is being produced in a cyclontron at a constant rate P by bombarding a manganese target with deutrons. .56Mn has a half-life of 2.5h and the target contains large numbers of only the stable manganese isotopes .56Mn. The reaction that produces .56Mn is
.56Mn+d→.56Mn+p
After being bombarded for a long time, the activity of .56Mn becomes constant, equal to 13.86×1010s−1. (Use 1n2=0.693, Avagardo number =6×102, atomic weight of .56Mn=56gmol−1).
At what constant rate P, .56Mn nuclei are being produced in the cyclontron during the bombardment?
View SolutionHelium nuclei combine to form an oxygen nucleus. The energy released in the reaction is if mO=15.9994amu and mHe=4.0026amu
View SolutionConsider a point sorce emiiting α- particles and receptor and receptor of area 1cm2 placed 1m away from source. Receptor records any α-particle falling on it. If the contains N0=3.0×1016 active nuclie and the receptor records a rate of A=50000counts/second. Assume that the source emits alpha particles unifomrly in all direction and the alpha particles fall nearly normally on the window. If decay constant is 3n×10−(n+1), then the value of n
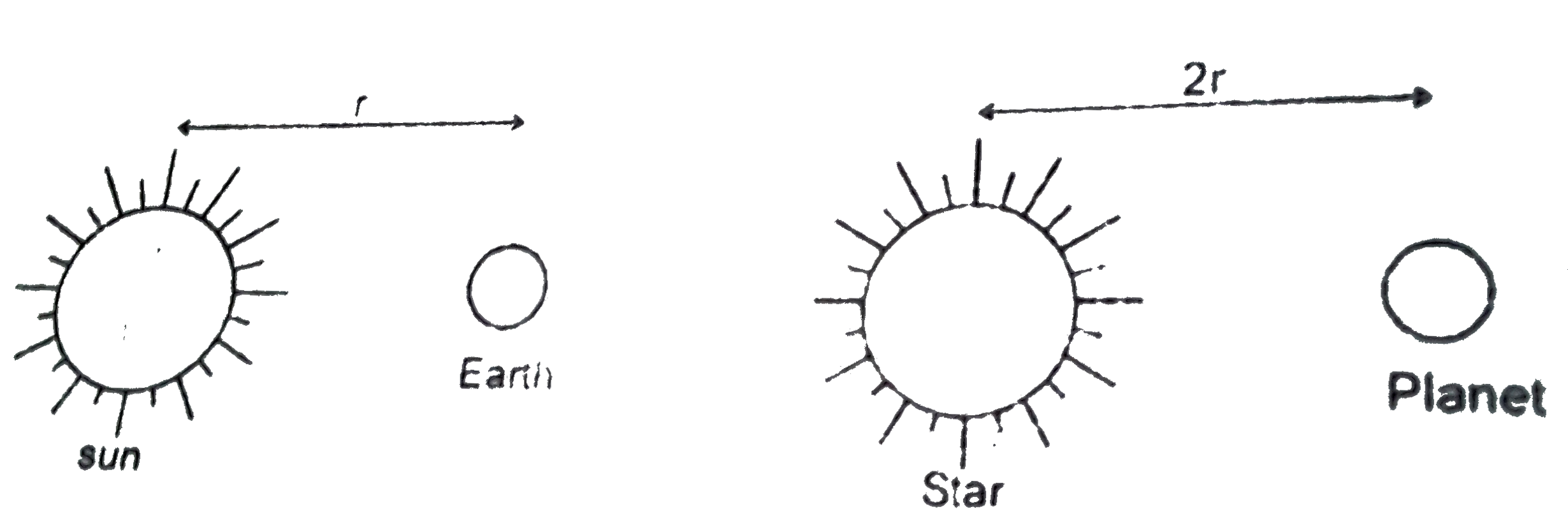
View SolutionLet a star be much brighter than our sun but its mass is same as that of sun If our earth has average lift span of man as 70years In the earth like planet of this star system having double the distance from our star find the average lift span of a man on this planet in terms of our year
 .
.
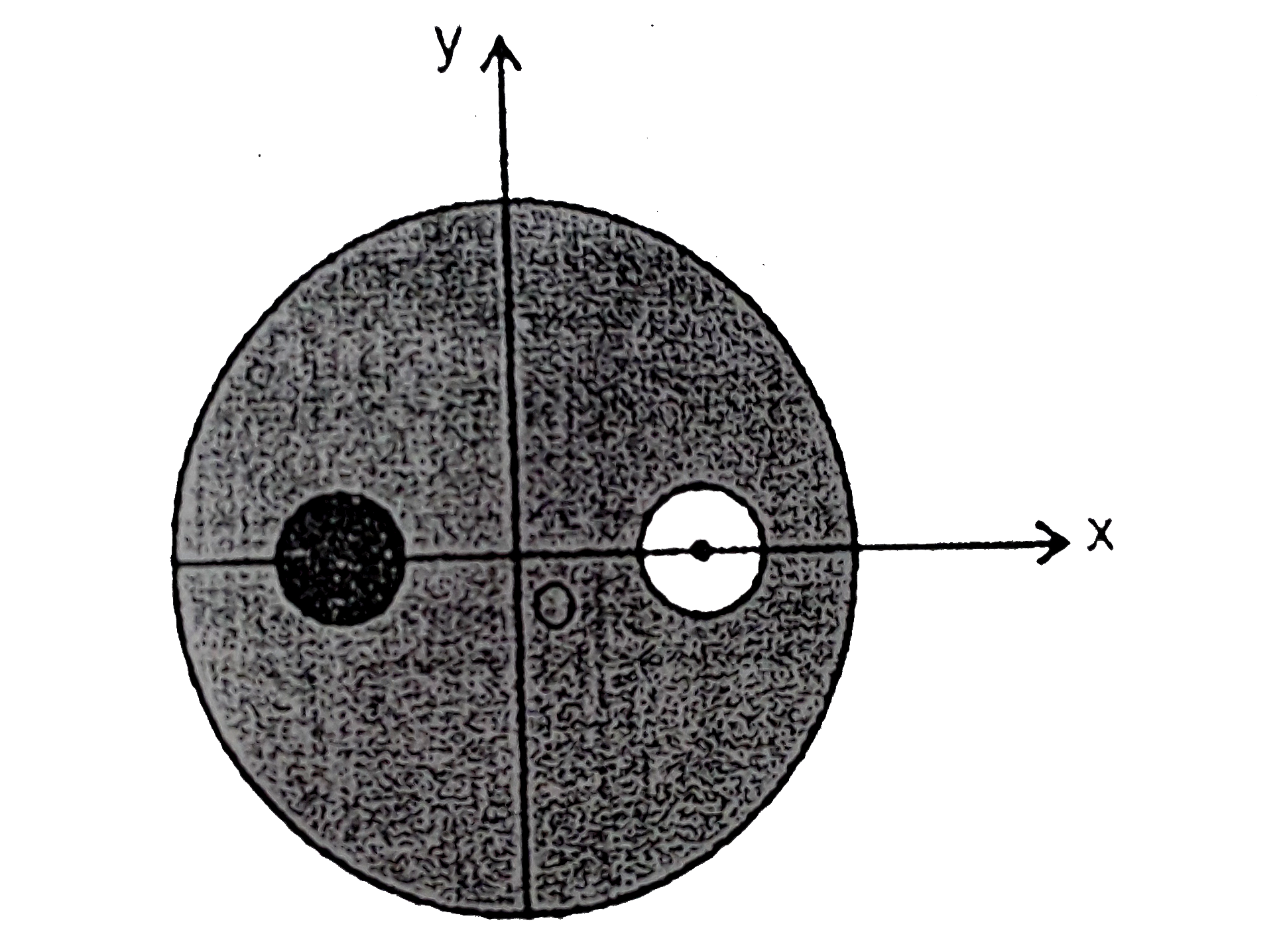
View SolutionConsider a solid sphere of density ρ and radius 4R. Centre of the sphere is at origin. Two spherical cavities centred at (2R,0) and (−2R,0) are created in sphere. Radii of both cavities is R. In left cavity material of density 2ρ is filled while second cavity is kept empty. What is gravitational field at origin.
View Solution.23892U has 92 protons and 238 nucleons. It decays by emitting an alpha particle and becomes:
View SolutionFind the half life of U238, if one gram of it emits 1.24×104 α-particle per second. Avogadro's Number =6.023×1023.
View SolutionA U^(238) preparation of mass 1.0g emits 1.24.10^(4) alpha particles per second. Find the life of this nuclide and the activity of the preparatio.
View Solution23892U emits an α-particle, the product has the atomic and mass numbers as
View Solution1.0 ग्राम U238 एक सेकण्ड में 1.24×104α−कण उतसर्जित करता है। U238 की अर्द्ध-आयु की गणना वर्षो में कीजिय। दिया है : आवोगाड्रो-संख्या=6×1023 प्रति मोल।
View SolutionAssertion :An α-particle is emitted when uranium 238 decays into thorium
Reason : The decay of uranium 238 to thorium is represented by .23892U→.23490Th+.42He. The helium nuclei is called an alpha particle.
View SolutionThe half-life of U238 is 4.5×109 y. In any radioactive sample if 1 g. of that element remains present then determine the activity of the sample in curie unit.
(Avogadro number = 6.023×1023).
View Solution23892U की अर्ध-आयु α- कण के विरुद्ध 1.5×1017 सेकंड है। 23892U के एक नमूने जिसमे 2.5×1020 परमाणु है, की सक्रियता क्या है ?
View Solution""_(92)U^(238) emits an alpha particle, the product has mass number and atomic number
View Solution.23892U में 92 प्रोटॉन तथा 238 न्यूक्लिऑन हैं | यह एक अल्फ़ा कण को उत्सर्जित करके क्षयित होता है तथा देता है
View SolutionA U238 preparation of mass 1 g emits 1.24×104α -particles per second. Find the half life of this nuclide and the activity of the preparation in becquerels:
View Solution
