A radioactive nucleus (initial mass number A and atomic number Z) emits 3 α-paticles and 2 positrons. The ratio of number of neutrons to that of protons in the final nucleus will be
A−Z−8Z−4
A−Z−4Z−8
A−Z−12Z−4
A−Z−4Z−2
The correct Answer is:B
.ZXA→32He4+.z−8YA−12+2+1e0+2υ
number of proton =Z−8
number of neutron =(A−12)−(Z−8)=A−Z−4
ratio is =A−Z−4Z−8
Topper's Solved these Questions
- NUCLEAR PHYSICSBook:RESONANCE ENGLISHChapter:NUCLEAR PHYSICSExercise:Exercise -3 Part-III CBSE PROBLEMS (LAST 10 YEARS)
Explore 34 Videos
- NUCLEAR PHYSICSBook:RESONANCE ENGLISHChapter:NUCLEAR PHYSICSExercise:Advanced level solutions
Explore 16 Videos
- NUCLEAR PHYSICSBook:RESONANCE ENGLISHChapter:NUCLEAR PHYSICSExercise:Exercise 3 Part -1 JEE (Advanced)
Explore 19 Videos
- GRAVITATIONBook:RESONANCE ENGLISHChapter:GRAVITATIONExercise:HIGH LEVEL PROBLEMS
Explore 16 Videos
- REVISION DPPBook:RESONANCE ENGLISHChapter:REVISION DPPExercise:All Questions
Explore 463 Videos
Similar Questions
When a deuteron of mass 2.0141 a.m.u and negligible K.E. is absorbed by a Lithium (.3Li6) nucleus of mass 6.0155 a.m.u. the compound nucleus disintegration spontaneously into two alpha particles, each of mass 4.0026 a.m.u. Calculate the energy carried by each α particle.
View SolutionA radioactive element decays by β−emission. A detector records n beta particles in 2s and in next 2s it records 0.75n beta particles. Find mean life correct to nearest whole number. Given ln |2|=0.6931, ln |3|=1.0986.
View SolutionHelium nuclei combine to form an oxygen nucleus. The energy released in the reaction is if mO=15.9994amu and mHe=4.0026amu
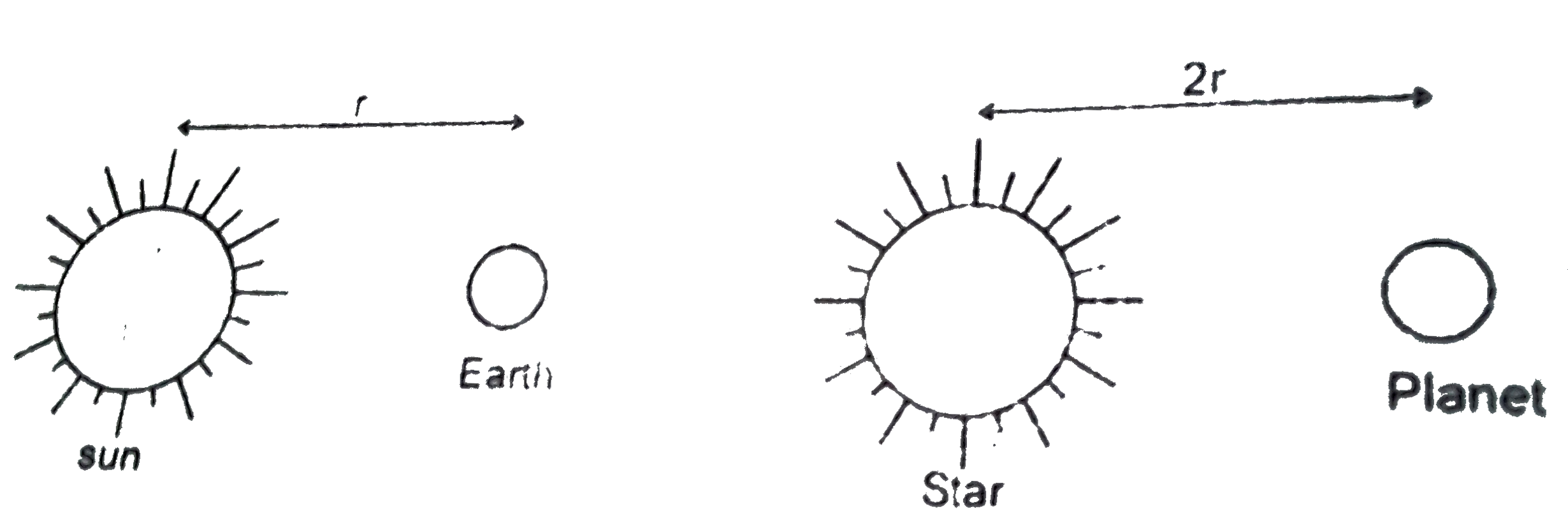
View SolutionLet a star be much brighter than our sun but its mass is same as that of sun If our earth has average lift span of man as 70years In the earth like planet of this star system having double the distance from our star find the average lift span of a man on this planet in terms of our year
 .
.
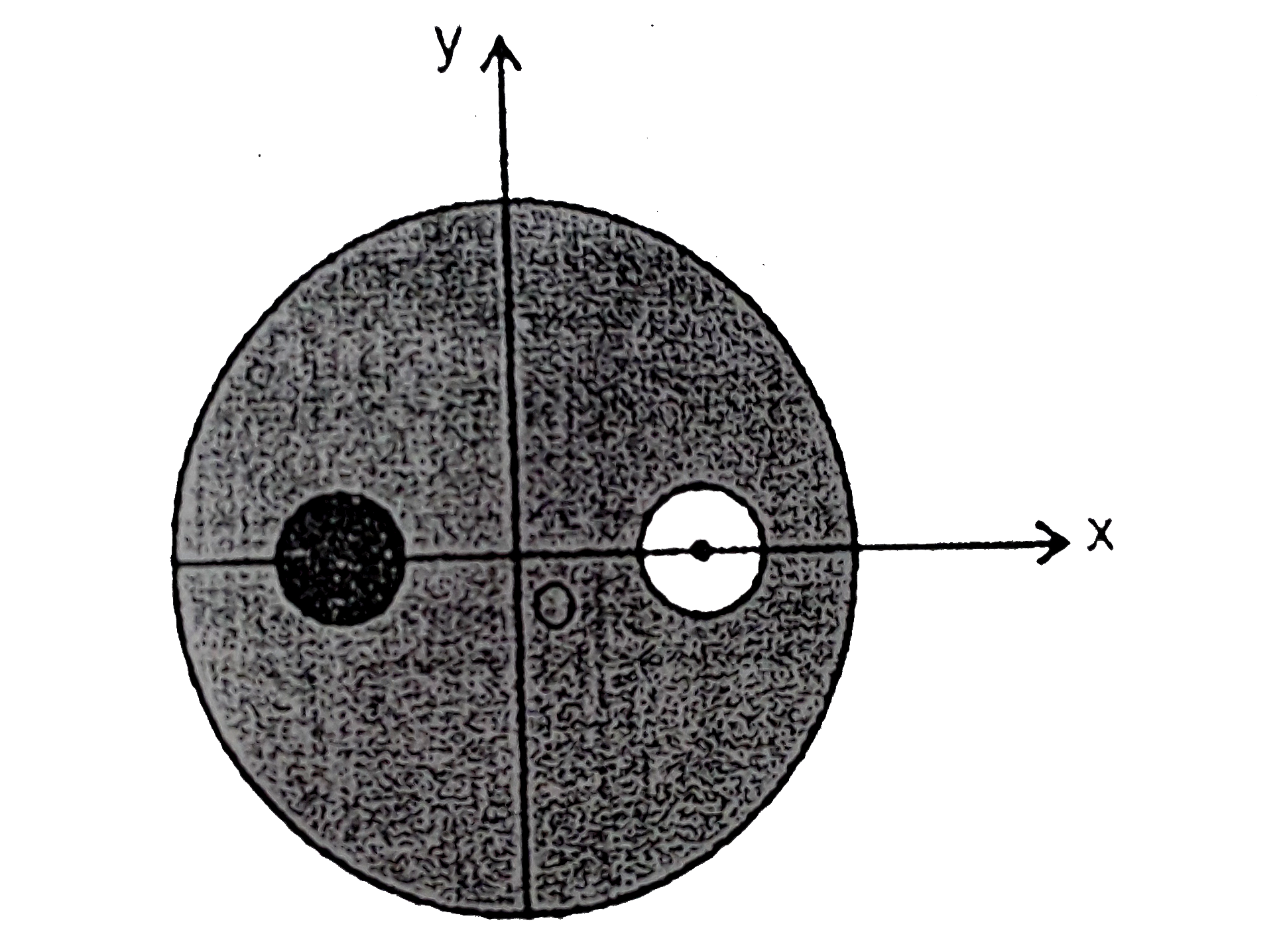
View SolutionConsider a solid sphere of density ρ and radius 4R. Centre of the sphere is at origin. Two spherical cavities centred at (2R,0) and (−2R,0) are created in sphere. Radii of both cavities is R. In left cavity material of density 2ρ is filled while second cavity is kept empty. What is gravitational field at origin.
View SolutionA radiaoactive nucleus (initial mass number A and atomic number Z emits 3 a- particles and 2 positrons The ratio of number of neutrons to that of proton in the final nucleus will be
View Solutionएक रेडियोऐक्टिव नाभिक (प्रारंभिक द्रव्यमान संख्या A तथा परमाणु क्रमांक Z) 3 अल्फा कण तथा 2 पाजिट्रॉन उत्सर्जित करता है | अंतिम नाभिक में न्यूट्रॉनों और प्रोटॉनों का अनुपात होगा -
View Solutionएक नाभिक, पोजिट्रॉन तथा γ विकिरण उत्सर्जित करता है | यदि प्रारम्भिक नाभिक के परमाणु क्रमांक व द्रव्यमान संख्या Z व A हैं तो अन्तिम नाभिक के परमाणु क्रमांक व द्रव्यमान संख्या होगी :
View Solutionएक नाभिक AZX, एक α-कण उत्सर्जित करता है और परिणामी नाभिक एक पोजिट्रॉन उत्सर्जित करता है | अन्तिम नाभिक का परमाणु क्रमांक व द्रव्यमान संख्या क्रमश: होगी:
View Solutionएक रेडियोऐक्टिव नाभिक (द्रव्यमान संख्या A तथा परमाणु संख्या Z) तीन α-कण तथा दो पोजिट्रॉन उत्सर्जित करता है | अन्तिम नाभिक मैं न्यूट्रॉनों तथा प्रोटॉनों की संख्या का अनुपात होगा :
View SolutionA radioactive nucleus (initial mass number A and atomic number Z) emits 3α-particles and 2 positrons. The ratio of number of neutrons to that of protons in the final nucleus will be
View SolutionA radioactive nucleus ._(Z) X^(A) emite 3 alpha- particles and 5 beta - particles . The ratio of number of neutrons to that of protons in the producet nucleus will be :
View SolutionA radioactive nucleus (initial mass number A and atomic number Z) emits 2α and 2 positrons. The ratio of number of neutrons to that of proton in the final nucleus will be
View SolutionA radioactive nucleus (initial mass number A and atomic number Z) emits 2α and 2 positrons. The ratio of number of neutrons to that of proton in the final nucleus will be
View SolutionA radioactive nucleus (initial mass number A and atomic number Z) emits 2α and 2 positrons. The ratio of number of neutrons to that of proton in the final nucleus will be
View SolutionA radioactive nucleus (initial mass number A and atomic number Z) emits 3 α -particles and 2 positrons. The ratio of number of neutrons to that of protons in the final nucleus will be:
View Solution
