(a) Write symbolically the β-decay process of 3215P.
(b) Derive an expression for the average life of a radionuclide . Give its relationship with the half-life.
(a) .3215P→.3216s+β−⇒.3215P→.3216S+.0−1e
(b) Average or Mean life: Average life is defined as the total life time of all the atoms of all the atoms of the element divided by the total number of atoms present in the sample of the element.
By definition average life time of the radioactive elements is
τ=N0∫0tdNN0
Since dN=−λN and N=N0e−λt
∴dN=−λN0e−λt
when N=0 the t=∝ and when N=N0
then t=0
∴τ0∫∝t−λN0e−λtdtN0=λ0∫−∝te−λtdt
τ=λ[te−λtλ−e−λtλ2] (Using intergration by parts)
τ=λ[(0−0)−(0−1λ2)]=λ×1λ2=1λ
Since λ=0.693t1/2
∴τ=10.693t1/2=t1/20.693=1.44t1/2
Hence the average life period of a radioactive element is 1.44 times tha half life peroid of the element
Topper's Solved these Questions
- NUCLEAR PHYSICSBook:RESONANCE ENGLISHChapter:NUCLEAR PHYSICSExercise:Advanced level solutions
Explore 16 Videos
- NUCLEAR PHYSICSBook:RESONANCE ENGLISHChapter:NUCLEAR PHYSICSExercise:Exercise-3 Part-II JEE (MAIN)
Explore 19 Videos
- GRAVITATIONBook:RESONANCE ENGLISHChapter:GRAVITATIONExercise:HIGH LEVEL PROBLEMS
Explore 16 Videos
- REVISION DPPBook:RESONANCE ENGLISHChapter:REVISION DPPExercise:All Questions
Explore 463 Videos
Similar Questions
A radioactive element decays by β−emission. A detector records n beta particles in 2s and in next 2s it records 0.75n beta particles. Find mean life correct to nearest whole number. Given ln |2|=0.6931, ln |3|=1.0986.
View SolutionStarting with a sample of pure 66Cu,78 of it decays into Zn in 15 min. The corresponding half-life is
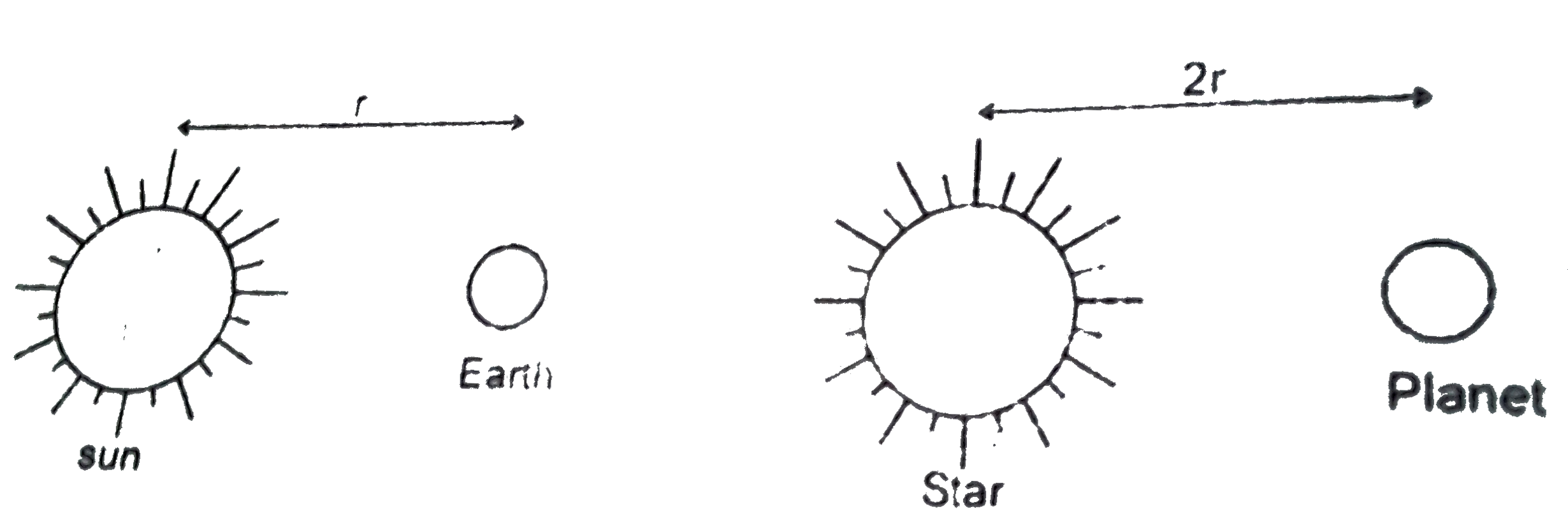
View SolutionLet a star be much brighter than our sun but its mass is same as that of sun If our earth has average lift span of man as 70years In the earth like planet of this star system having double the distance from our star find the average lift span of a man on this planet in terms of our year
 .
.
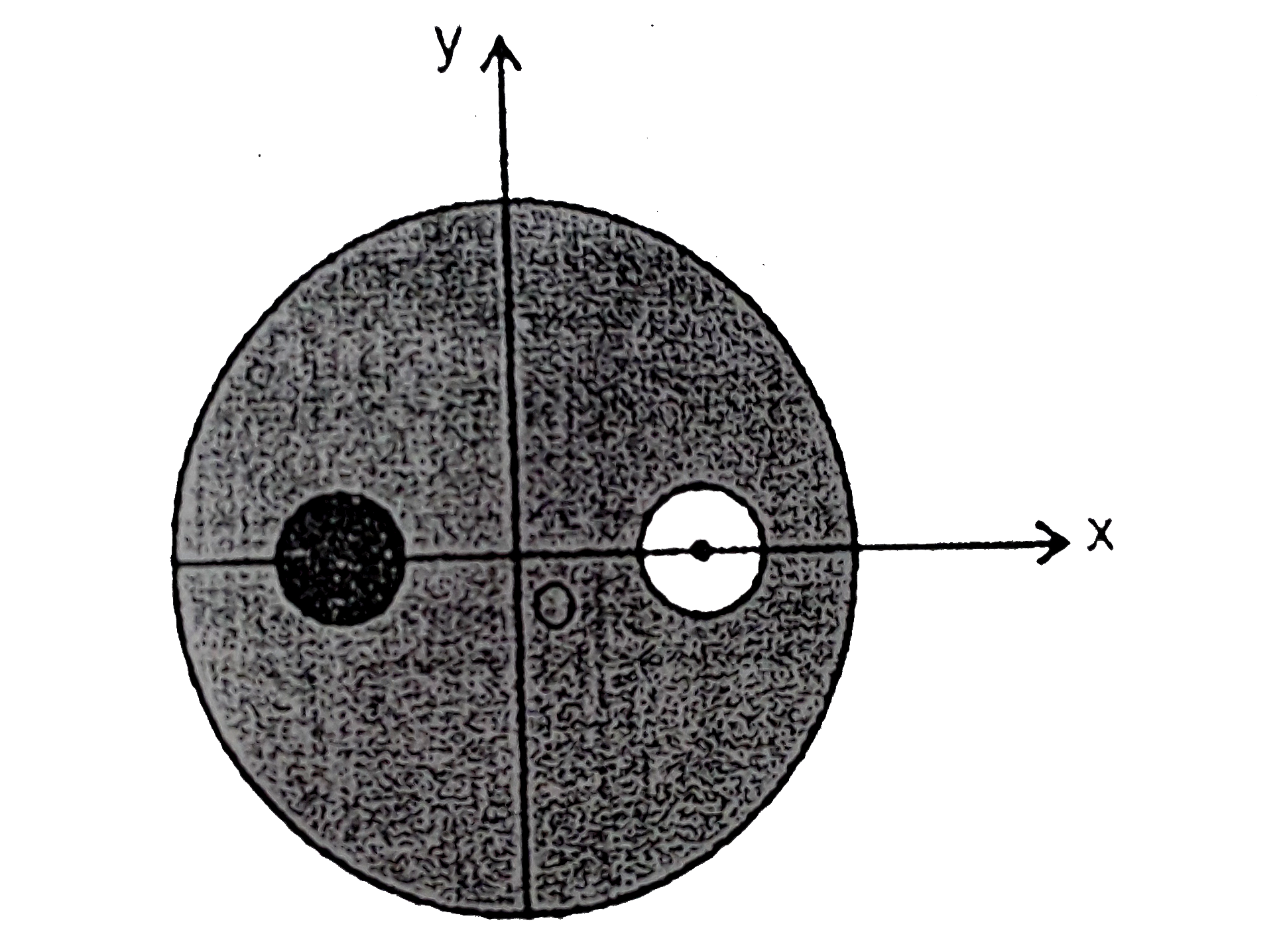
View SolutionConsider a solid sphere of density ρ and radius 4R. Centre of the sphere is at origin. Two spherical cavities centred at (2R,0) and (−2R,0) are created in sphere. Radii of both cavities is R. In left cavity material of density 2ρ is filled while second cavity is kept empty. What is gravitational field at origin.
View SolutionThe half-life of a radionuclide is 69.3 minutes. What is its average life (in minutes)
View Solution(a) Write symbolically the β-decay process of 3215P.
(b) Derive an expression for the average life of a radionuclide . Give its relationship with the half-life.
View SolutionWrite symbolically the process expressing the β+ decay of .2211Na. Also write the basic nuclear process underlying this decay.
View SolutionCalculate the average life (in minutes ) , if the half - life of a radionuclide is 69.3 . Minutes
View SolutionWhat is meant by average life of a radioactive element ? Derive an expression for it. What is the relation between average life and half life ?
View Solution3215P की नाभिकीय क्षय प्रक्रिया लिखें।
View Solution(a) 3215P की β - क्षय प्रक्रिया , प्रतीकात्मक रूप में लिखें।
View Solution(a) Write symbolically the beta -decay process of phosphorus. (b) Derive an expression for the average life of a radionuclide. Give its relationship with the half-life.
View SolutionWrite the nuclear decay process for β−-decay of 3215P.
View Solution(a) Write symbolically the β− decay process of 3215P.
(b) Derive an expression for the average life of a radionuclide. Give its relationship with the half-life.
View Solution(a) Write symbolically the beta -decay process of ""_(15)^(32)P . (b) Derive an expression for the average life of a radionuclide . Give its relationship with the half-life.
View SolutionWhat is meany by average life of a radioactive element? Derive an expression for it. What is the relation between average life and half life?
View Solution
