Simple six memebered ring compound (eg. Cyclohexane) are not soluble in water whereas glucose and sucrose are soluble in water. Explain why ?
The correct Answer is:Glucose has five −OH group and sucrose has then −OH group and because of hydrogen bonding with H2O molecules glucose and sucrose are soluble in water.
To explain why simple six-membered ring compounds like cyclohexane are not soluble in water, while glucose and sucrose are soluble, we can break down the explanation into several steps:
Step 1: Understanding the Structures
- Cyclohexane is a simple six-membered carbon ring (C6H12) that consists entirely of carbon and hydrogen atoms. Its structure is non-polar because it lacks any functional groups that can interact with water.
- Glucose (C6H12O6) and sucrose (C12H22O11) are both carbohydrates that contain multiple hydroxyl (–OH) groups in their structures. These hydroxyl groups are polar.
Step 2: Polarity of Compounds
- Cyclohexane is non-polar due to its hydrocarbon nature. It does not have any polar bonds or functional groups that can interact with water molecules.
- Glucose and sucrose, on the other hand, are polar molecules because of the presence of hydroxyl groups. These –OH groups can form hydrogen bonds with water.
Step 3: Solubility Principle
- The principle of solubility states that "like dissolves like." This means that polar substances tend to dissolve in polar solvents, while non-polar substances do not.
- Water (H2O) is a polar solvent, which means it can dissolve other polar substances effectively.
Step 4: Interaction with Water
- Glucose and sucrose can form hydrogen bonds with water due to their hydroxyl groups. This interaction allows them to dissolve in water.
- Cyclohexane, being non-polar, does not interact with water molecules and thus remains insoluble.
Conclusion
In summary, the solubility of glucose and sucrose in water is attributed to their polar nature and the presence of hydroxyl groups that allow for hydrogen bonding with water. In contrast, cyclohexane is non-polar and does not interact with water, making it insoluble.
---
- Cyclohexane is a simple six-membered carbon ring (C6H12) that consists entirely of carbon and hydrogen atoms. Its structure is non-polar because it lacks any functional groups that can interact with water.
- Glucose (C6H12O6) and sucrose (C12H22O11) are both carbohydrates that contain multiple hydroxyl (–OH) groups in their structures. These hydroxyl groups are polar.
- Cyclohexane is non-polar due to its hydrocarbon nature. It does not have any polar bonds or functional groups that can interact with water molecules.
- Glucose and sucrose, on the other hand, are polar molecules because of the presence of hydroxyl groups. These –OH groups can form hydrogen bonds with water.
- The principle of solubility states that "like dissolves like." This means that polar substances tend to dissolve in polar solvents, while non-polar substances do not.
- Water (H2O) is a polar solvent, which means it can dissolve other polar substances effectively.
- Glucose and sucrose can form hydrogen bonds with water due to their hydroxyl groups. This interaction allows them to dissolve in water.
- Cyclohexane, being non-polar, does not interact with water molecules and thus remains insoluble.
In summary, the solubility of glucose and sucrose in water is attributed to their polar nature and the presence of hydroxyl groups that allow for hydrogen bonding with water. In contrast, cyclohexane is non-polar and does not interact with water, making it insoluble.
Topper's Solved these Questions
- BIOMOLECULESBOOK - RESONANCE ENGLISHCHAPTER - BIOMOLECULESEXERCISE - Ex-1(sec-B)Part-I
8 Videos
- BIOMOLECULESBOOK - RESONANCE ENGLISHCHAPTER - BIOMOLECULESEXERCISE - Ex-1(sec-C)Part-I
5 Videos
- BIOMOLECULESBOOK - RESONANCE ENGLISHCHAPTER - BIOMOLECULESEXERCISE - Advanced Level(Part II)
33 Videos
- BASIC CONCEPTSBOOK - RESONANCE ENGLISHCHAPTER - BASIC CONCEPTSEXERCISE - ORGANIC CHEMISTRY(BASIC CONCEPTS)
27 Videos
- BIOMOLECULES & POLYMERBOOK - RESONANCE ENGLISHCHAPTER - BIOMOLECULES & POLYMEREXERCISE - ORGANIC CHEMISTRY(Biomolecules & Polymer)
34 Videos
Similar Questions
The melting point and solubility (inH2O) of amino acids are generally high. Explain why ?
View SolutionExplain the difference between Buna-N and Buna-S.
View SolutionIf rate of diffusion of A is 6 times that of B, what will be the density ratio of A and B?
View SolutionThe kinetic energy of an electron accelerated from rest through a potential difference of 3V will be:
View SolutionWhich two of the following aldohexoses give the same osazone derivative ?
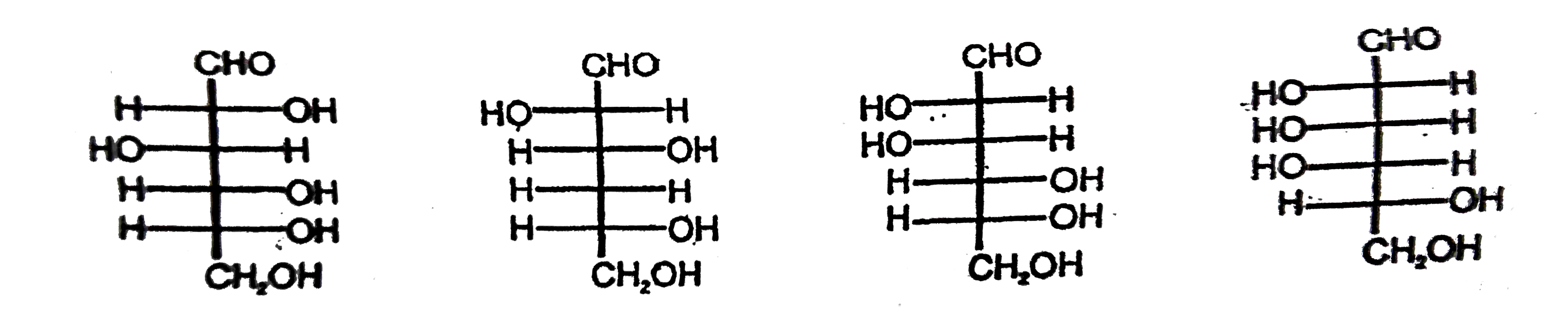
View SolutionGlucose or sucrose are soluble in water but cyclohexane or bezene (simple six membered ring compounds) are insoluble in water. Explain.
View SolutionSimple six memebered ring compound (eg. Cyclohexane) are not soluble in water whereas glucose and sucrose are soluble in water. Explain why ?
View Solutionग्लूकोस तथा सुक्रोस जल में विलेय हैं जबकि साइक्लोहेक्सेन अथवा बेंजीन (सामान्य छः सदस्यीय वलय युक्त यौगिक) जल में अविलेय होते हैं । समझाइए ।
View SolutionWhy glucose, fructose, sucrose etc. are soluble in water through they are covalent compounds ?
View Solutionग्लूकोज तथा सुक्रोज जल में विलेय हैं जबकि लाइक्लोहेक्सेन अथवा बेंजीन ( सामान्य छः सदस्यीय वलय युक्त यौगिक ) जल में अविलेय होता है। क्यों ?
View SolutionGlucose or sucrose are soluble in water but cyclohexane and benzene (simple six membered ring compound are insoluble in water. Explain.
View SolutionGlucose or sucrose are soluble in water but cyclohexane or bezene (simple six membered ring compounds) are insoluble in water. Explain.
View Solutionग्लूकोज तथा स्यूक्रोज जल में विलेय है, परन्तु साइक्लोहेक्सेन या बेंजीन (सरल 6 समदायी वलय योगिक) जल में अविलेय है, क्यों ?
View SolutionThrough benzene is water-insoluble, glucose and sucrose are water-soluble. Why?
View SolutionGlucose or sucrose are soluble in water but cyclohexane or benzene are insoluble in water. Explain
View SolutionGlucose or sucrose are soluble in water but cyclohexane and benzene are insolule in water. Explain.
View Solution

