The rms speed of helium gas at 27∘C and 1 atm pressure is 600ms−1. Then the rms speed of helium molecules at temperature 27∘C and 2 atm pressure is
100ms−1
400ms−1
600ms−1
800ms−1
The correct Answer is:B
To solve the problem, we need to understand the relationship between the root mean square (rms) speed of a gas and its properties. The formula for calculating the rms speed (v_rms) of a gas is given by:
vrms=√3RTM
Where:
- R is the universal gas constant,
- T is the absolute temperature in Kelvin,
- M is the molar mass of the gas.
1. Identify the Given Information:
- The rms speed of helium gas at 27∘C and 1 atm is 600m/s.
- The temperature remains constant at 27∘C (which is 300K).
- The molar mass of helium is constant.
- The pressure changes from 1 atm to 2 atm.
2. Understand the Effect of Pressure on rms Speed:
- The formula for rms speed does not include pressure as a variable. It depends only on temperature and molar mass.
- Since the temperature and molar mass of helium remain constant, the rms speed will not change with the change in pressure.
3. Conclusion:
- Therefore, the rms speed of helium molecules at 27∘C and 2 atm pressure will still be 600m/s.
Final Answer:
The rms speed of helium molecules at 27∘C and 2 atm pressure is 600m/s.
---
- R is the universal gas constant,
- T is the absolute temperature in Kelvin,
- M is the molar mass of the gas.
- The rms speed of helium gas at 27∘C and 1 atm is 600m/s.
- The temperature remains constant at 27∘C (which is 300K).
- The molar mass of helium is constant.
- The pressure changes from 1 atm to 2 atm.
- The formula for rms speed does not include pressure as a variable. It depends only on temperature and molar mass.
- Since the temperature and molar mass of helium remain constant, the rms speed will not change with the change in pressure.
- Therefore, the rms speed of helium molecules at 27∘C and 2 atm pressure will still be 600m/s.
The rms speed of helium molecules at 27∘C and 2 atm pressure is 600m/s.
Topper's Solved these Questions
- BIOMOLECULESBook:RESONANCE ENGLISHChapter:BIOMOLECULESExercise:Ex-3(IIT-JEE)Part-I
Explore 18 Videos
- BIOMOLECULESBook:RESONANCE ENGLISHChapter:BIOMOLECULESExercise:Ex-3(IIT-JEE)Part-II
Explore 15 Videos
- BIOMOLECULESBook:RESONANCE ENGLISHChapter:BIOMOLECULESExercise:Ex-2(More correct)Part-II
Explore 14 Videos
- BASIC CONCEPTSBook:RESONANCE ENGLISHChapter:BASIC CONCEPTSExercise:ORGANIC CHEMISTRY(BASIC CONCEPTS)
Explore 27 Videos
- BIOMOLECULES & POLYMERBook:RESONANCE ENGLISHChapter:BIOMOLECULES & POLYMERExercise:ORGANIC CHEMISTRY(Biomolecules & Polymer)
Explore 34 Videos
Similar Questions
Aspartame, an artificial sweetener, is peptide and has the following structure :
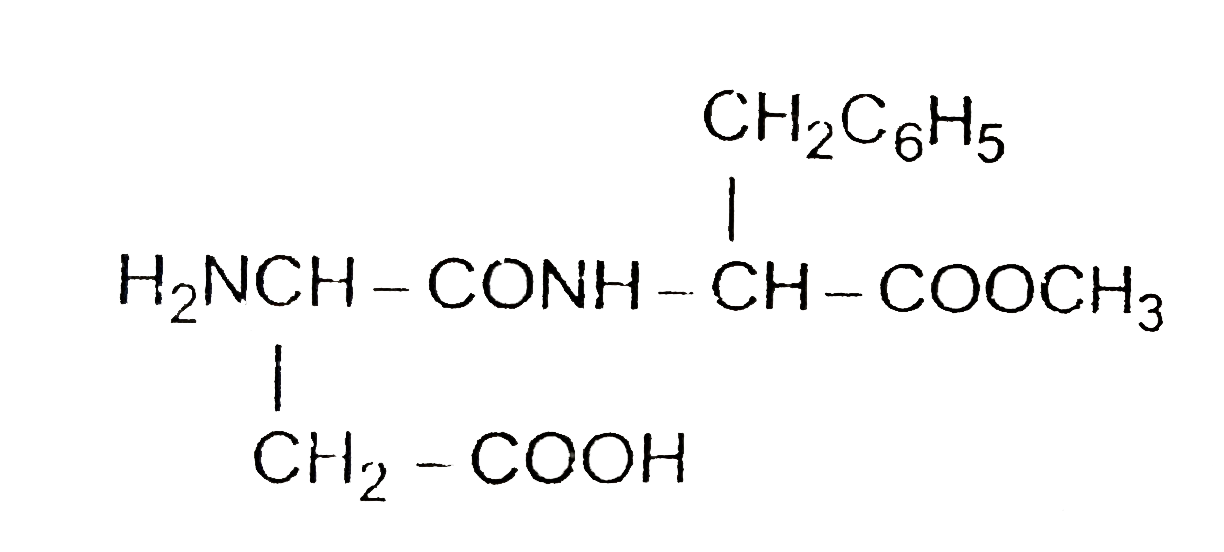 .
.
View SolutionA substance forms zwitter ion, it can have functional groups −
View SolutionThe rms speed of helium gas at 27∘C and 1 atm pressure is 1100ms−1. Then the rms speed of helium molecules at temperature 27∘C and 2 atm pressure is
View SolutionThe kinetic energy of an electron accelerated from rest through a potential difference of 3V will be:
View SolutionWhich two of the following aldohexoses give the same osazone derivative ?
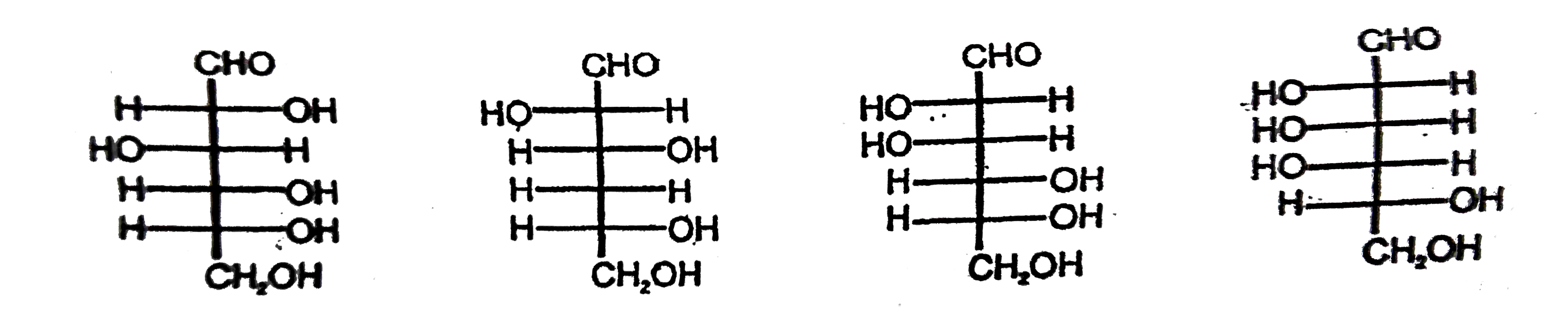
View SolutionThe rms speed of helium gas at 27∘C and 1 atm pressure is 900 ms−1. Then the rms speed of helium molecules at temperature 27∘C and 2 atm pressure is
View SolutionFor a given gas at 1 atm pressure, rms speed of the molecules is 200 m/s at 127∘C. At 2 atm pressure and at 227∘C, the rms speed of the molecules will be:
View SolutionThe molecules of a given mass of gas have an rms velocity of 200 ms(-1) at 27^@C and pressure 1 atm. When the temperature is 127^@C and pressure is 2 atm, the rms velocity in m s^(-1) will be ?
View SolutionFor a given gas at 1 atm pressure, rms speed of the molecules is 200m/s at 127∘C. At 2 atm pressure and at 227∘C, the speed of the molecules will be :
View SolutionRMS speed of gas molecule at 27^oC and 1 atm pressure is 200 m/s and rms speed at 127^oC and 2 atm pressure is (200/sqrt3)x .Find value of x
View SolutionThe rms speed of helium at 24^(@)C and 1 atm pressure is 450ms^(-1) . Then the rms speed of the helium molecules at 24^(@)C and 2 atm pressure is
View SolutionThe rms speed of helium gas at 27∘C and 1 atm pressure is 900ms−1. Then the rms speed of helium molecules at temperature 27∘C and 2 atm pressure is
View SolutionThe rms speed of helium gas at 27∘C and 1 atm pressure is 800ms−1. Then the rms speed of helium molecules at temperature 27∘C and 2 atm pressure is
View SolutionThe rms speed of helium gas at 27^(@)C and 1 atm pressure is 1100 ms^(-1) . Then the rms speed of helium molecules at temperature 27^(@)C and 2 atm pressure is
View SolutionThe rms speed of helium gas at 27^(@)C and 1 atm pressure is 500 ms^(-1) . Then the rms speed of helium molecules at temperature 27^(@)C and 2 atm pressure is
View Solution
