The following diagram shows the arrangement of lattice points with a=b=c and α=β=γ=90∘. Choose the correct options. 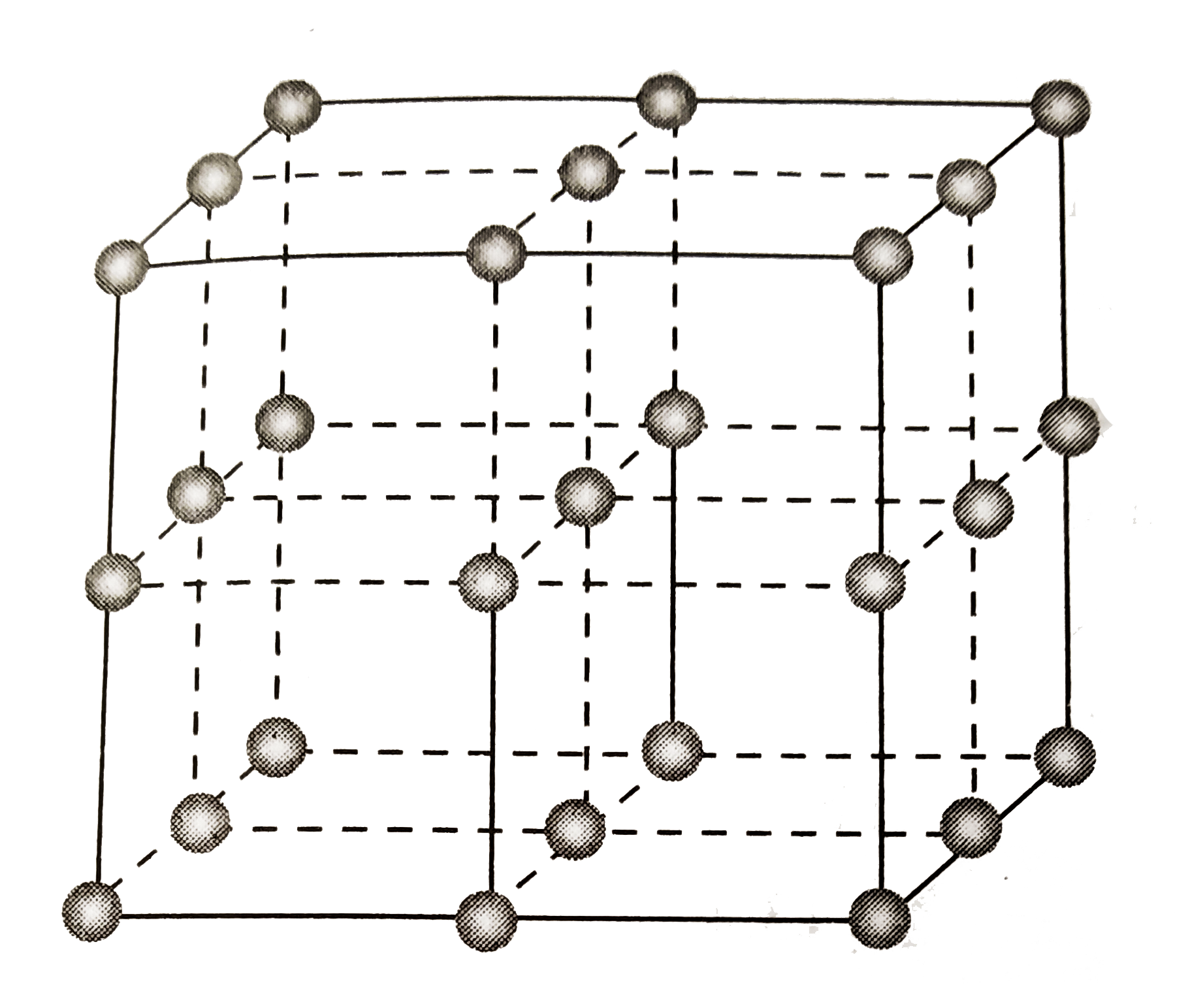
The arrangement is SC with each lattice point surrounded by 6 nearest neighbours.
The arrangement is SC with each lattice point surrounded by 8 nearest neighbouts.
The arrangement is FCC with each lattice point surrounded by 12 nearest neighbours.
The arrangement in BCC with each lattice point surrounded by 8 nearest neighbours.
The correct Answer is:1
According to figure, it shows a simple cubic lattice. Now observe the centre atom, its has 6 nearest neighbours.
Topper's Solved these Questions
Similar Questions
The reduction potential of hydrogen half cell will be negative if :
View SolutionA system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vapourizes to form a more disordered gas. When a solute is present, there is additional contribution to the entropy of the liquid due to increased randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas. Thus, a solute (non-volatile) lowers the vapour pressure of a liquid, and hence a higher boiling point of the solution.
Similarly, the greater randomness of the solution opposes the tendercy to freeze. In consequence, a lower temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution. The elevation in boiling point (ΔTb) and depression in freezing point (ΔTf) of a solution are the colligative properties which depend only on the concentration of particles of the solute and not their identity. For dilute solutions, (ΔTb) and (ΔTf) are proportional to the molarity of the solute in the solution.
To aqueous solution of Nal, increasing amounts of solid Hgl2 is added. The vapour pressure of the solution
View SolutionThe following diagram shows the arrangement of lattice points with a=b=c and α=β=γ=90∘. Choose the correct options.

View SolutionArrange in increasing order of:
a. The mass of α,β, and γ
b. The penetration power of α,β, and γ
c. The speed of α,β, and γ
d. The inoization capacity of gases of α,β, and γ
View Solutionif a=b ne c and alpha=beta=gamma=90^(@) , the crystal system is
View SolutionThe unit cell with parameters alpha=beta=gamma=90^(@) and a=b!=c is :-
View SolutionThe crystal system for which a!= b!= c and alpha = beta = gamma = 90^(@) is said to be
View SolutionIf a != b != c and alpha = beta = gamma = 90^(º) , the crystal system is
View SolutionThe relation a=b=candα=β=γ=90∘belong to the _______crystal system
View SolutionIdentify the following unit cell: (1) a=bnec, alpha=beta=gamma=90^(@) (2) anebnec, alpha=beta=gamma=90^(@)
View SolutionA crystal system a = b ! = c and alpha = beta = gamma = 90. The crystal system is ________.
View Solutionif a=b ne c and alpha=beta=gamma=90^(@) , the crystal system is
View SolutionThe crystal system for which a!= b!= c and alpha = beta = gamma = 90^(@) is said to be
View SolutionThe unit cell with crystallographic dimensions a=b≠c,α=β=γ=90∘ is
View SolutionThe unit cell with crystallographic dimensions =b!=c,alpha=beta=gamma=90^@ is:
View SolutionThe following diagram shows the arrangement of lattice points with a=b=c and α=β=γ=90∘. Choose the correct options.

View Solution

