Copper has a face− centred cubic structure with a unit− cell edge length of 3.61Å. What is the size of the largest atom which could fit into the intersectices of the copper lattice without distorting if ?
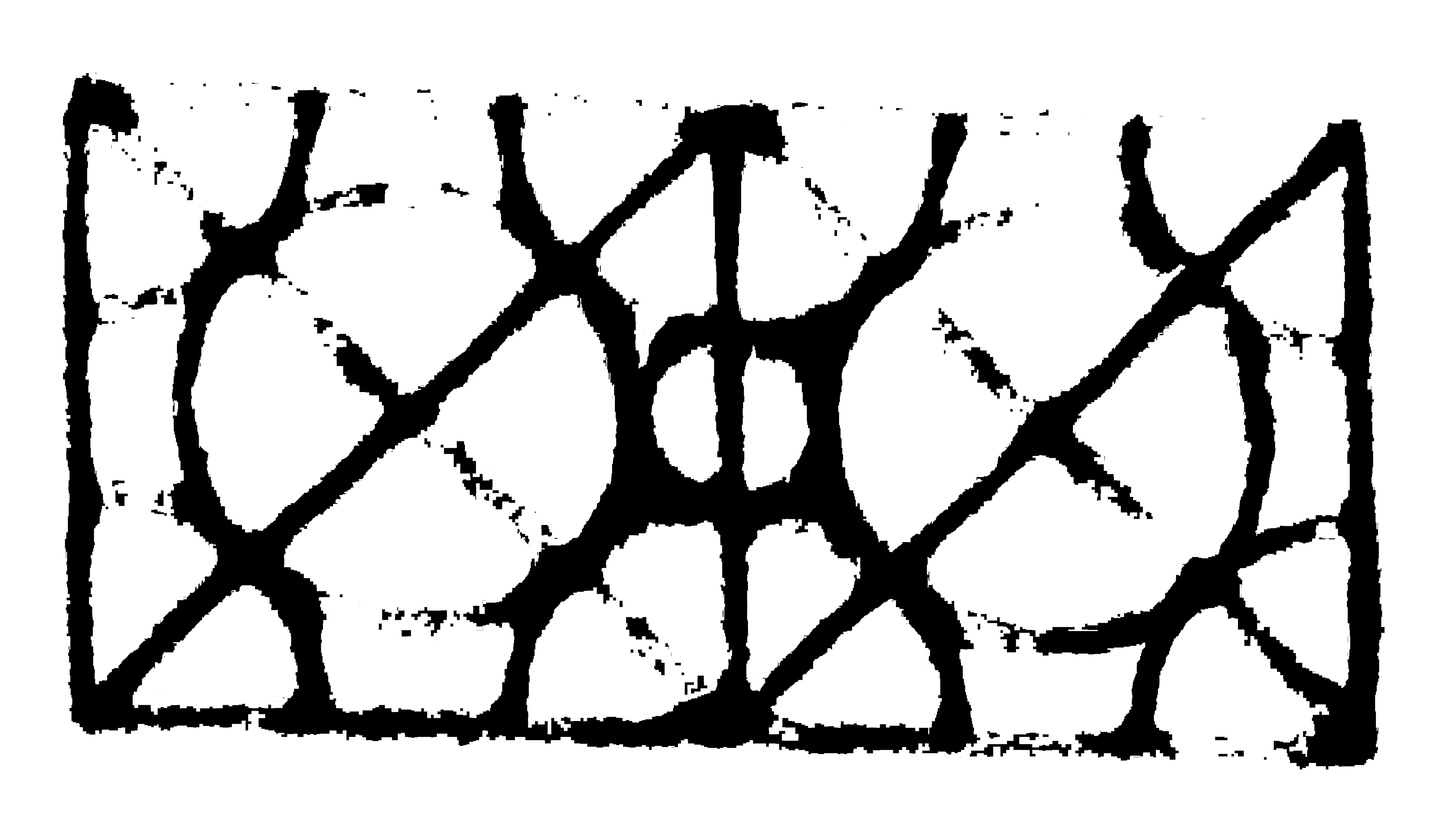
0.53Å
0.33Å
0.58Å
0.13Å
The correct Answer is:1
roctahedral=0.414R
for FCC 4R=√2a
R=√2a4
r=0.414√2a4=0.414√2×3.614=0.53Å
Topper's Solved these Questions
Similar Questions
The reduction potential of hydrogen half cell will be negative if :
View SolutionA system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vapourizes to form a more disordered gas. When a solute is present, there is additional contribution to the entropy of the liquid due to increased randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas. Thus, a solute (non-volatile) lowers the vapour pressure of a liquid, and hence a higher boiling point of the solution.
Similarly, the greater randomness of the solution opposes the tendercy to freeze. In consequence, a lower temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution. The elevation in boiling point (ΔTb) and depression in freezing point (ΔTf) of a solution are the colligative properties which depend only on the concentration of particles of the solute and not their identity. For dilute solutions, (ΔTb) and (ΔTf) are proportional to the molarity of the solute in the solution.
To aqueous solution of Nal, increasing amounts of solid Hgl2 is added. The vapour pressure of the solution
View SolutionCopper crystalline in a face centred cubic lattice with a unit cell length of 361pm .What is the radius of copper atom in p m?
View SolutionCopper has a face− centred cubic structure with a unit− cell edge length of 3.61Å. What is the size of the largest atom which could fit into the intersectices of the copper lattice without distorting if ?

View SolutionCopper crystallises in a structure of face centred cubic unit cell. The atomic radius of copper is 1.28Å. What is axial length on an edge of copper?
View SolutionThe radius of copper atom is 128 pm. It it crystallises in face centred cubic lattice (fcc), what will be the length the edge of the unit cell ?
View SolutionCopper crystallises in face-centred cubic lattice with a unit cell length of 361 pm. What is the radius of copper atom in pm?
View SolutionCopper crystallises in a structure of face centerd cubic unit cell. The atomic radius of copper is .28Å. What is axial length on an edge of copper ?
View SolutionCopper crystallises in a face-centred cubic lattice. Molar mass and density of copper are 63.5g*mol^(-1)and8.9g*cm^(-3) respectively. Calculate the edge length of a unit cell in copper lattice and the radius of a copper atom.
View SolutionIn face centred cubic (fcc) crystal lattice edge length of the unit cell is 400 pm. Find the diameter fo the greatest sphere which can be fitted into the interstitial void without the distortion of lattice.
View SolutionCopper crystallizes in a face-centred cublic lattice with a unit cell length of 361pm. What is the radius of copper atom in pm ?
View SolutionCopper crystallizes in a face centred cubic lattice. Calculate the number of unit cells in 1.2g of copper. Atomic mass of copper = 63.54.
View SolutionCopper crystallizes in a face centred cubic lattice. Calculate the number of unit cells in 1.2g of copper. Atomic mass of copper = 63.54.
View SolutionCopper crystallises in a structure of face centerd cubic unit cell. The atomic radius of copper is 1.28 Å . What is axial length on an edge of copper ?
View SolutionCopper crystallises in face-centred cubic lattice with a unit cell length of 361 pm. What is the radius of copper atom in pm?
View Solution
