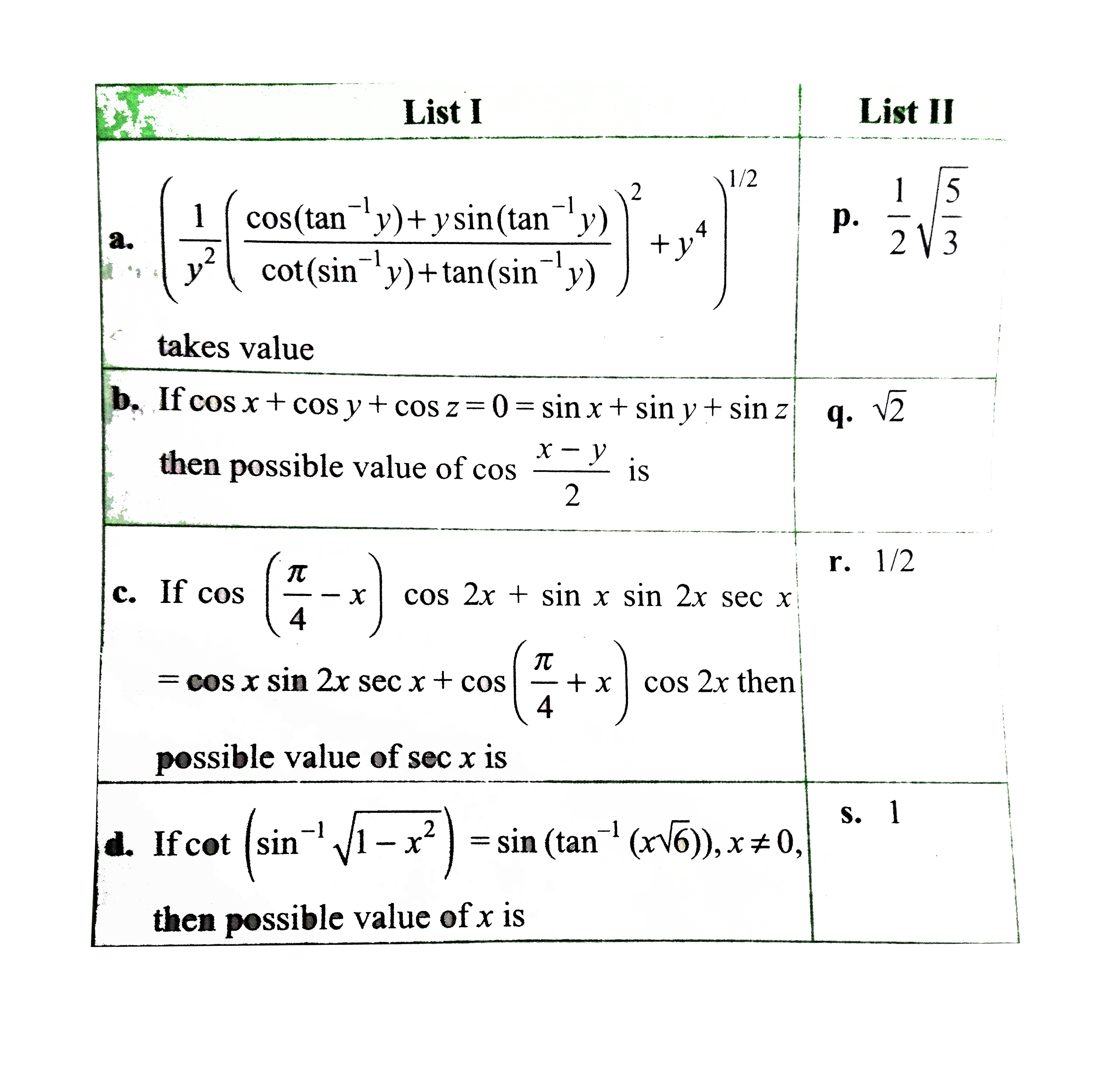
Match the List -I (containing anions ) with List -II (containing reagent used in testing ) using the codes as given below in the column.
List−IList−II(anions)(reagents)(a)S2−(p)Barium chloride solution in presence of HCl(b)NO−3(q)Sodium nitroprusside(c)I−(r)chlorine water and chloroform(d)SO2−4(s)iron(II)sulphate solution and conc.H2SOS4
Code :
(a)(b)(c)(d)(q)(s)(r)(p)
(a)(b)(c)(d)(p)(s)(r)(q)
(a)(b)(c)(d)(q)(p)(r)(s)
(a)(b)(c)(d)(p)(q)(r)(s)
The correct Answer is:1
To match the anions from List-I with the appropriate reagents from List-II, we will analyze each anion and determine the correct testing reagent based on the information provided.
1. Identify the Anion: Sulfide Ion (S²⁻)
- Reagent: Sodium nitroprusside
- Explanation: The presence of sulfide ions can be detected by treating the sample with sodium nitroprusside, which forms a violet-colored complex.
- Match: (a) S²⁻ → (q) Sodium nitroprusside
2. Identify the Anion: Nitrate Ion (NO₃⁻)
- Reagent: Iron(II) sulfate solution and concentrated sulfuric acid
- Explanation: For nitrate ions, when treated with freshly prepared iron(II) sulfate and concentrated sulfuric acid, a brown ring complex is formed at the junction of the two liquids, indicating the presence of nitrate ions.
- Match: (b) NO₃⁻ → (s) Iron(II) sulfate solution and concentrated H₂SO₄
3. Identify the Anion: Iodide Ion (I⁻)
- Reagent: Chlorine water and chloroform
- Explanation: The presence of iodide ions can be detected using chlorine water, which oxidizes iodide ions to iodine (I₂). The iodine dissolves in chloroform, forming a violet layer.
- Match: (c) I⁻ → (r) Chlorine water and chloroform
4. Identify the Anion: Sulfate Ion (SO₄²⁻)
- Reagent: Barium chloride solution in presence of HCl
- Explanation: When sulfate ions are treated with barium chloride, a white precipitate of barium sulfate (BaSO₄) is formed, indicating the presence of sulfate ions.
- Match: (d) SO₄²⁻ → (p) Barium chloride solution in presence of HCl
Final Matches:
- (a) S²⁻ → (q) Sodium nitroprusside
- (b) NO₃⁻ → (s) Iron(II) sulfate solution and concentrated H₂SO₄
- (c) I⁻ → (r) Chlorine water and chloroform
- (d) SO₄²⁻ → (p) Barium chloride solution in presence of HCl
Codes:
- A: q
- B: s
- C: r
- D: p
- Reagent: Sodium nitroprusside
- Explanation: The presence of sulfide ions can be detected by treating the sample with sodium nitroprusside, which forms a violet-colored complex.
- Match: (a) S²⁻ → (q) Sodium nitroprusside
- Reagent: Iron(II) sulfate solution and concentrated sulfuric acid
- Explanation: For nitrate ions, when treated with freshly prepared iron(II) sulfate and concentrated sulfuric acid, a brown ring complex is formed at the junction of the two liquids, indicating the presence of nitrate ions.
- Match: (b) NO₃⁻ → (s) Iron(II) sulfate solution and concentrated H₂SO₄
- Reagent: Chlorine water and chloroform
- Explanation: The presence of iodide ions can be detected using chlorine water, which oxidizes iodide ions to iodine (I₂). The iodine dissolves in chloroform, forming a violet layer.
- Match: (c) I⁻ → (r) Chlorine water and chloroform
- Reagent: Barium chloride solution in presence of HCl
- Explanation: When sulfate ions are treated with barium chloride, a white precipitate of barium sulfate (BaSO₄) is formed, indicating the presence of sulfate ions.
- Match: (d) SO₄²⁻ → (p) Barium chloride solution in presence of HCl
- (a) S²⁻ → (q) Sodium nitroprusside
- (b) NO₃⁻ → (s) Iron(II) sulfate solution and concentrated H₂SO₄
- (c) I⁻ → (r) Chlorine water and chloroform
- (d) SO₄²⁻ → (p) Barium chloride solution in presence of HCl
- A: q
- B: s
- C: r
- D: p
Topper's Solved these Questions
Similar Questions
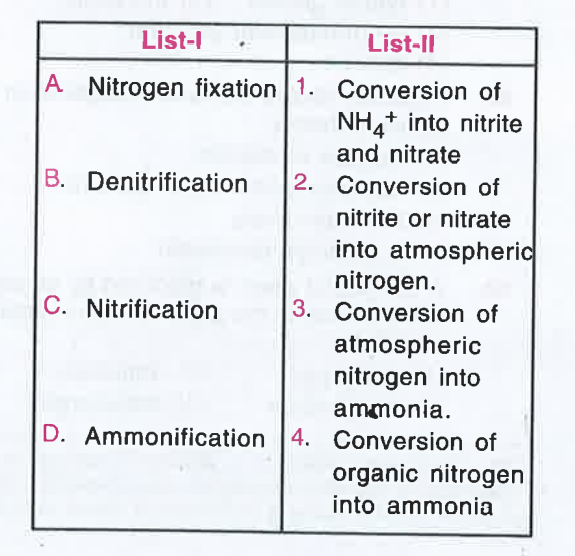
Knowledge Check
Match List I with List II and select the correct answer by using the codes given below the lists:
 AI-D, II - A, III -B, IV-CBI-A, II – D, III -C, IV-BCI-A, II – D, III – B, IV-CDI-D, II -C, III – B, IV-A
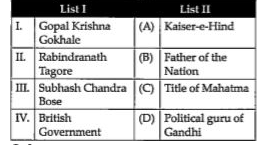
AI-D, II - A, III -B, IV-CBI-A, II – D, III -C, IV-BCI-A, II – D, III – B, IV-CDI-D, II -C, III – B, IV-AMatch List I with List II and select the correct answer by using the codes given below the lists:

 AI-D, II - A, III - C, IV-BBI-B, II - D, III-A, IV-CCI-A, II – D, III – B, IV-CDI-D, II-A, III -B, IV-C
AI-D, II - A, III - C, IV-BBI-B, II - D, III-A, IV-CCI-A, II – D, III – B, IV-CDI-D, II-A, III -B, IV-CMatch List I with List II and select the correct answer by using the codes given below the lists:
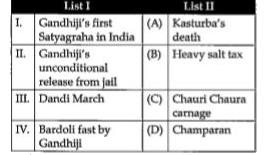 AI-D, II – A, III - C,IV-BBI-A, II-D, III -C, IV-BCI-A, II -D, III - B, IV-CDI-D, II-A, III -B, IV-C
AI-D, II – A, III - C,IV-BBI-A, II-D, III -C, IV-BCI-A, II -D, III - B, IV-CDI-D, II-A, III -B, IV-C
Match List -I with List -II and select the correct answer using the codes given below the lists

View SolutionMatch List I with List II and select the correct answer using the codes given below the lists
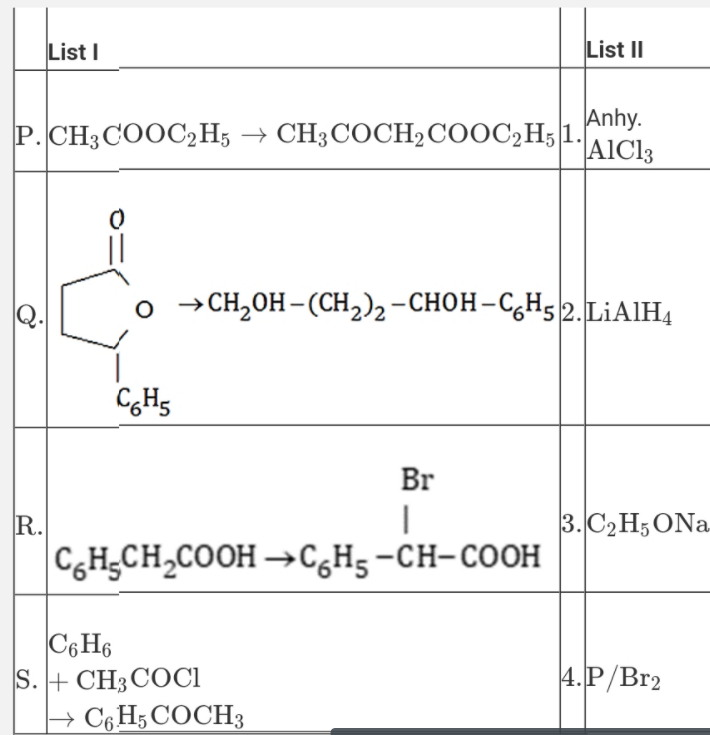
View SolutionMatch List I with List II and select the correct answer by using the codes given below the lists:
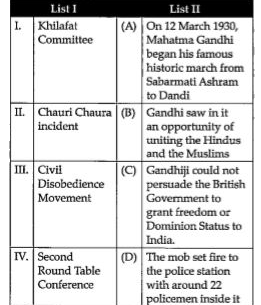
View SolutionMatch List I with List II and select the correct answer by using the codes given below the lists:
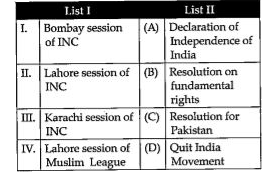
View SolutionMatch List I with List II and select the correct answer by using the codes given below the lists:
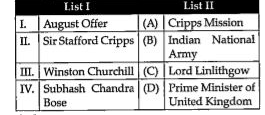
View Solution