In which of the following compound Mn shoes minimum radius ?
MnO2
KMnO4
MnO
None of these
The correct Answer is:3
To determine in which compound manganese (Mn) shows the minimum radius, we need to analyze the oxidation states of Mn in the given compounds and understand how oxidation state affects ionic radius.
1. Identify the Compounds: The compounds given are MnO2, KMnO4, and MnO.
2. Determine the Oxidation States of Mn:
- For MnO2:
- Let the oxidation state of Mn be x.
- The oxidation state of oxygen is -2.
- The equation becomes:
x+2(−2)=0⟹x−4=0⟹x=+4
- For KMnO4:
- Let the oxidation state of Mn be x.
- The oxidation state of potassium (K) is +1 and the oxidation state of oxygen is -2.
- The equation becomes:
1+x+4(−2)=0⟹1+x−8=0⟹x−7=0⟹x=+7
- For MnO:
- Let the oxidation state of Mn be x.
- The oxidation state of oxygen is -2.
- The equation becomes:
x+(−2)=0⟹x+2=0⟹x=+2
3. Compare the Oxidation States:
- Mn in MnO2 has an oxidation state of +4.
- Mn in KMnO4 has an oxidation state of +7.
- Mn in MnO has an oxidation state of +2.
4. Analyze the Effect of Oxidation State on Ionic Radius:
- The higher the oxidation state of a metal ion, the smaller its ionic radius due to increased effective nuclear charge.
- Therefore, Mn in KMnO4 (+7) will have the smallest radius compared to Mn in MnO2 (+4) and MnO (+2).
5. Conclusion:
- Since Mn has the highest oxidation state (+7) in KMnO4, it will show the minimum radius in this compound.
Final Answer:
Mn shows the minimum radius in the compound KMnO4.
- For MnO2:
- Let the oxidation state of Mn be x.
- The oxidation state of oxygen is -2.
- The equation becomes:
x+2(−2)=0⟹x−4=0⟹x=+4
- For KMnO4:
- Let the oxidation state of Mn be x.
- The oxidation state of potassium (K) is +1 and the oxidation state of oxygen is -2.
- The equation becomes:
1+x+4(−2)=0⟹1+x−8=0⟹x−7=0⟹x=+7
- For MnO:
- Let the oxidation state of Mn be x.
- The oxidation state of oxygen is -2.
- The equation becomes:
x+(−2)=0⟹x+2=0⟹x=+2
- Mn in MnO2 has an oxidation state of +4.
- Mn in KMnO4 has an oxidation state of +7.
- Mn in MnO has an oxidation state of +2.
- The higher the oxidation state of a metal ion, the smaller its ionic radius due to increased effective nuclear charge.
- Therefore, Mn in KMnO4 (+7) will have the smallest radius compared to Mn in MnO2 (+4) and MnO (+2).
- Since Mn has the highest oxidation state (+7) in KMnO4, it will show the minimum radius in this compound.
Mn shows the minimum radius in the compound KMnO4.
Topper's Solved these Questions
- PERIODIC TABLE & PERIODICITYBook:RESONANCE ENGLISHChapter:PERIODIC TABLE & PERIODICITYExercise:Inorganic chemistry (Periodic Table & Periodicity
Explore 57 Videos
- PERIODIC TABLE & PERIODICITYBook:RESONANCE ENGLISHChapter:PERIODIC TABLE & PERIODICITYExercise:ORGANIC CHEMISTRY(BASIC CONCEPTS)
Explore 24 Videos
- ORGANIC REACTION MECHANISMS-IVBook:RESONANCE ENGLISHChapter:ORGANIC REACTION MECHANISMS-IVExercise:APSP PART-3
Explore 22 Videos
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBook:RESONANCE ENGLISHChapter:REDUCTION, OXIDATION & HYDROLYSIS REACTIONSExercise:Comprehension Type
Explore 3 Videos
Similar Questions
An element with atomic number 107 has recently been discovered. Its block, group number, period and outshell electronic configuration respectively are :
View SolutionAn element with atomic number 107 has recently been discovered. Its block, group number, period and outshell electronic configuration respectively are :
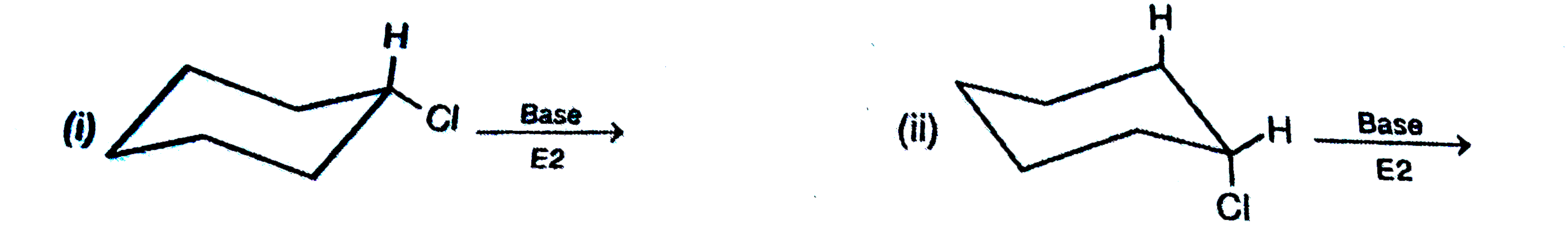
View SolutionRate of Bimolecular elimination (E2) reaction for the following :
View SolutionColour of ppt formed when HgCl2 reacts with KI is
View SolutionIn which of the following compound Mn shoes minimum radius ?
View SolutionThe ionic radius of Cr is minimum in which of the following compounds ?
View SolutionIn which of the following compounds, Mn has highest oxidation state?
View SolutionIn which of the following compound Mn shows maximum radius?
View SolutionIn follwing compound which has minimum ionic radius of maganese is :
View Solutionनिम्न में से किसकी त्रिज्या न्यूनतम है?
View SolutionMn की अधिकतम ऑक्सीकरण संख्या निम्न में से कौन-से यैगिक में प्रदर्शित होती है ?
View SolutionThe ionic radius of Cr is minimum in which the following compounds ?
View SolutionThe ionic radius of 'Cr' is minimum in which of the following compounds-
View Solutionनिम्न आयनों में कौनसा न्यूनतम त्रिज्या वाला है
View Solutionनिम्न में से किस योगिक में Mn की ऑक्सीकरण संख्या का मान सर्वाधिक होता है
View Solution
