What is the basicity of H3PO4?
The structure of H3PO4 is tetrahedral. It has three P−OH and one P=O bond as shown below:
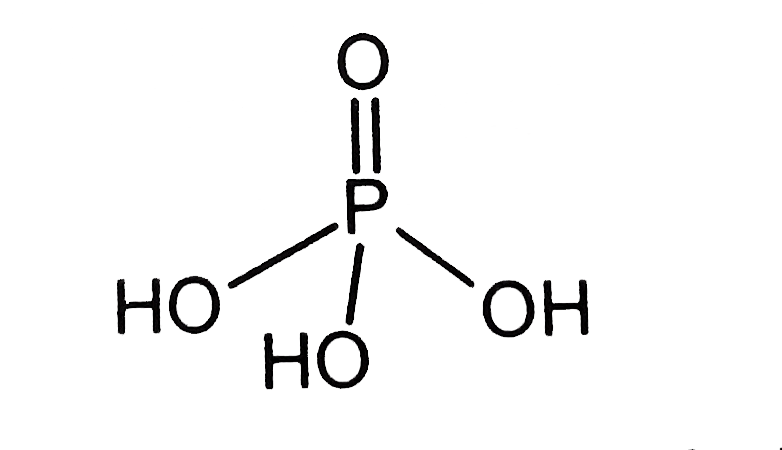
Since it is three ionizable P−OH bonds.
Topper's Solved these Questions
- P BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:P BLOCK ELEMENTSExercise:Exercise 1 part 1 subjective ques
Explore 30 Videos
- P BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:P BLOCK ELEMENTSExercise:Exercise 1 part 2 objective que
Explore 47 Videos
- P BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:P BLOCK ELEMENTSExercise:Example
Explore 30 Videos
- NUCLEAR CHEMISTRYBook:RESONANCE ENGLISHChapter:NUCLEAR CHEMISTRYExercise:STAGE-II
Explore 1 Video
- P-BLOCK ELEMENT (BORON AND CARBON FAMILY)Book:RESONANCE ENGLISHChapter:P-BLOCK ELEMENT (BORON AND CARBON FAMILY)Exercise:PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3
Explore 8 Videos
Similar Questions
What is the basicity of H3PO3 ?
View SolutionWhat is the basicity of H3PO3 ?
View SolutionWhat is the basicity of H3PO2 acid and why ?
View SolutionWhat is the sum of basicity of H3PO4,H3PO3,H3PO2,H3BO3,(COOH)2,H2S2O8,H2SO4,H2SO3,H2CO3,H2S and H4P2O5?
View SolutionWhat is the n-factor for H3PO4 in the reaction 2NaOH+H3PO4→Na2HPO4+2H2O?
View SolutionThe normality of 1.5MH3PO4 is-
View SolutionThe pH of blood is 7.4 . What is the ratio of [HPO2−4H2PO−4] in the blood. pKa(H2PO−4)=7.1
View SolutionThe true statement of the acids of phosphorus H3PO2,H3PO3 and H_(3)PO4 is :
View SolutionKa1,Ka2,Ka3 values for H3PO4 are 10−3,10−8 and 10−12 respectively .If Kw(H2O)=10−14 then ,
(i) What is dissociation constant of HPO2−4?
(ii) What is Kb of HPO2−4
(iii) What is Kb of H2PO−4?
(iv) What is order of Kb of Po3−4(Kb3),HPO2−4(Kb2) and H2PO−4(Kb1)?
View SolutionCalculate equivalent weight of H3PO4 and Ca(OH)2 on the basis of given reaction.
H3PO4+NaOH→NaH2PO4+H2O
Ca(OH)2+HCL→Ca(OH)CL+H2O
View Solution
