Why do nitro compounds have high boiling points in comparison with other compounds of same molecular mass ?
Video Solution
Text Solution
Nitro compounds are highly polar in nature, therefore, there is strong electrostatic, attraction between nitrogen and oxygen atoms of a nitro group. As a result, a large amount of energy is requried to break th nitrogen and oxygen nonds. Hence, nitro comupounds have high boiling points in comarison with other comopounds of same molecular mass. 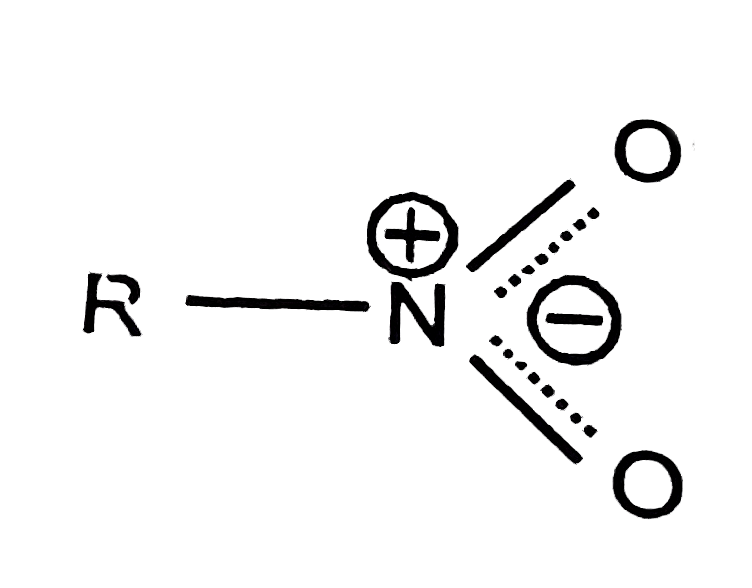
|
Topper's Solved these Questions
- P BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:P BLOCK ELEMENTSExercise:ALP PART 1 SUBJECTIVE
Explore 3 Videos
- P BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:P BLOCK ELEMENTSExercise:ALP PART II AIEEE OBJECTIVE
Explore 1 Video
- P BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:P BLOCK ELEMENTSExercise:ALP EX #3 OBJECTIVE
Explore 1 Video
- NUCLEAR CHEMISTRYBook:RESONANCE ENGLISHChapter:NUCLEAR CHEMISTRYExercise:STAGE-II
Explore 1 Video
- P-BLOCK ELEMENT (BORON AND CARBON FAMILY)Book:RESONANCE ENGLISHChapter:P-BLOCK ELEMENT (BORON AND CARBON FAMILY)Exercise:PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3
Explore 8 Videos
Similar Questions
Knowledge Check
The compound with the highest boiling point is
An-hexaneBn-pentaneC2,2-dimethylpropaneD2-methylbutane.
Which compound has the highest boiling point?
View SolutionThe compound with the highest boiling point is
View SolutionThe compound with the highest boiling point is:
View SolutionThe compound with the highest boiling point is:
View SolutionWhy do ionic compounds possess high melting and boiling points ?
View SolutionWhy do noble gases have low boiling points ?
View SolutionExplain the following:
lonic compounds have high melting point and boiling point while covalent compounds have low melting and boiling points.
View Solution
