The ratio of kinetic energy and potential energy of an electron in a Bohr orbit of a hydrogen− like species is :
1/2
−1/2
1
−1
The correct Answer is:2
Step by step video solution for The ratio of kinetic energy and potential energy of an electron in a Bohr orbit of a hydrogen- like species is : by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- TEST PAPERSBook:RESONANCE ENGLISHChapter:TEST PAPERSExercise:FST test -2
Explore 30 Videos
- TEST PAPERSBook:RESONANCE ENGLISHChapter:TEST PAPERSExercise:FST-3
Explore 30 Videos
- TEST PAPERSBook:RESONANCE ENGLISHChapter:TEST PAPERSExercise:PT-06
Explore 28 Videos
- SURFACE CHEMISTRYBook:RESONANCE ENGLISHChapter:SURFACE CHEMISTRYExercise:Section - 5
Explore 1 Video
- TEST SERIESBook:RESONANCE ENGLISHChapter:TEST SERIESExercise:CHEMISTRY
Explore 50 Videos
Similar Questions
A first order reaction takes 40 min for 30% decomposition. What will be t1/2?
View SolutionUltraviolet light of 5.2eV falls on Aluminium surface (work function = 3.2eV). The kinetic energy in joules of the fastest electron emitted is approximately:
View SolutionWhich of the following compoud on hydrolysis gives acetylene ?
View SolutionMatch list I with list II and select the correct answer :
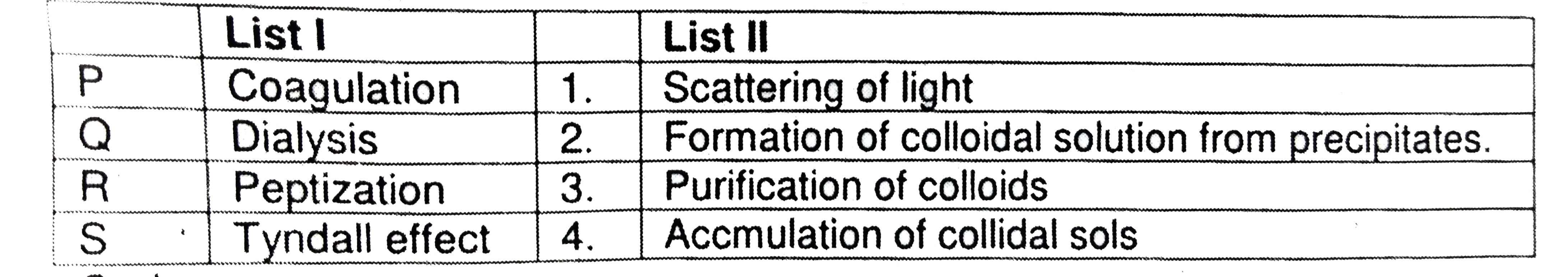
View Solutionwhich of the following plots will obtained for a conductometric titration of strong acid against a weak base?
View SolutionThe ratio of kinetic energy and potential energy of an electron in a Bohr of a hydrogen -like species is
View SolutionThe ratio of kinetic energy and total energy of an electron in a Bohr of a hydrogen like species is
View SolutionThe ratio of potential energy and total energy of an electron in a Bohr of a hydrogen -like species is
View SolutionThe ratio of kinetic energy to the total energy of an electron in a Bohr orbit of the hydrogen atom, is
View SolutionThe ratio of kinetic energy and potential energy of an electron in a Bohr orbit of a hydrogen - like species is :
View SolutionThe ratio of kinetic energy of the total energy of an electron in a Bohr orbit of the hydrogen atom, is
View SolutionThe ratio of kinetic energy to the total energy of an electron in a Bohr orbit of the hydrogen atom is
View Solutionहाइड्रोजन परमाणु की किसी बोर कक्षा में इलेक्ट्रॉन की गतिज ऊर्जा का स्थितिज ऊर्जा से अनुपात होता है :
View SolutionThe ratio of potential energy and total energy of an electron in a Bohr orbit of a hydrogen -like species is
View SolutionThe ratio of kinetic energy to the total energy of an electron in a Bohr orbit of the hydrogen atom is
View SolutionIn any Bohr orbit of the hydrogen atom, the ratio of kinetic energy to potential energy of the electron is ......
View Solution
