An alpha particle after passing through a potential difference of 3×106 volt falls on a silver foil. The atomic number of silver is 47. The K.E. of the α-particle at the time of falling on the foil is:
9.6×10−13J
4.5×10−13J
5.6×10−13J
1.5×10−13J
The correct Answer is:1
To find the kinetic energy (K.E.) of the alpha particle after passing through a potential difference of 3×106 volts, we can follow these steps:
Step 1: Understand the relationship between potential difference and kinetic energy
The kinetic energy gained by a charged particle when it moves through a potential difference V is given by the formula:
K.E.=q⋅V
where q is the charge of the particle and V is the potential difference.
Step 2: Determine the charge of the alpha particle
An alpha particle consists of 2 protons and 2 neutrons. Since neutrons do not carry charge, the charge of the alpha particle is:
q=2⋅e
where e (the elementary charge) is approximately 1.6×10−19 coulombs. Therefore:
q=2⋅(1.6×10−19)=3.2×10−19 coulombs
Step 3: Calculate the kinetic energy in electron volts
Using the potential difference V=3×106 volts, we can calculate the kinetic energy in electron volts:
K.E.=q⋅V=2⋅(3×106)=6×106 electron volts
Step 4: Convert kinetic energy from electron volts to joules
To convert the kinetic energy from electron volts to joules, we use the conversion factor 1 eV=1.6×10−19 J:
K.E.=6×106 eV×1.6×10−19 J/eV=9.6×10−13 J
Final Answer
Thus, the kinetic energy of the alpha particle at the time of falling on the foil is:
K.E.=9.6×10−13 J
---
The kinetic energy gained by a charged particle when it moves through a potential difference V is given by the formula:
K.E.=q⋅V
where q is the charge of the particle and V is the potential difference.
An alpha particle consists of 2 protons and 2 neutrons. Since neutrons do not carry charge, the charge of the alpha particle is:
q=2⋅e
where e (the elementary charge) is approximately 1.6×10−19 coulombs. Therefore:
q=2⋅(1.6×10−19)=3.2×10−19 coulombs
Using the potential difference V=3×106 volts, we can calculate the kinetic energy in electron volts:
K.E.=q⋅V=2⋅(3×106)=6×106 electron volts
To convert the kinetic energy from electron volts to joules, we use the conversion factor 1 eV=1.6×10−19 J:
K.E.=6×106 eV×1.6×10−19 J/eV=9.6×10−13 J
Thus, the kinetic energy of the alpha particle at the time of falling on the foil is:
K.E.=9.6×10−13 J
Topper's Solved these Questions
- TEST PAPERSBook:RESONANCE ENGLISHChapter:TEST PAPERSExercise:FST-3
Explore 30 Videos
- TEST PAPERSBook:RESONANCE ENGLISHChapter:TEST PAPERSExercise:FST 01
Explore 30 Videos
- SURFACE CHEMISTRYBook:RESONANCE ENGLISHChapter:SURFACE CHEMISTRYExercise:Section - 5
Explore 1 Video
- TEST SERIESBook:RESONANCE ENGLISHChapter:TEST SERIESExercise:CHEMISTRY
Explore 50 Videos
Similar Questions
Ultraviolet light of 5.2eV falls on Aluminium surface (work function = 3.2eV). The kinetic energy in joules of the fastest electron emitted is approximately:
View SolutionA sodium street light gives off yellow light that has a wavelength of 600nm. Then .
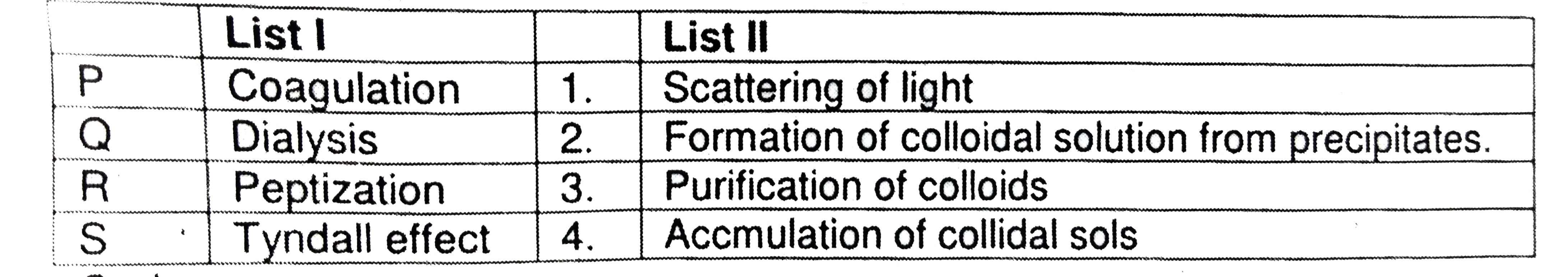
View SolutionMatch list I with list II and select the correct answer :
View Solutionwhich of the following plots will obtained for a conductometric titration of strong acid against a weak base?
View Solutionalpha- particles of 6 MeV energy is scattered back form a silver foil. Calculate the maximum volume in which the entire positive charge fo the atom is supposed to be concentrated. ( Z for silver = 47 )
View SolutionAn α-particle is accelerated through a.p.d of 106 volt the K.E. of particle will be
View SolutionA narrow beam of alpha particles falls normally on a silver foil behind which a counter is set to register the scattered particles. On substraction of platinum foil of the same mass thickness for the silver foil, the number of alpha particles registered per unit time increased eta= 1.52 time s . Find the atomic number of platinum, assuming the atomic number of silver and the atomic masses of both platinum and silver to be known.
View SolutionAn alpha particle after passing through a potential difference of 2xx10^(6) volt falls ona silver foil. The atomic number of silver is 47. Calculate (i) the K.E. of the alpha-particle at the time of falling on the foil. (ii) K.E. of the alpha -particle at a distance of 5xx10^(-14)m from the nucleus, (iii) the shortest distance from the nucleus of silver to which the alpha -particle reaches.
View SolutionImagine that in any atom about 50% of the space is occupied by the atomic nucleus . If a silver foil is bombarded with alpha - particles , majority of the alpha -particles would :
View SolutionAn alpha -particle after passing through a potential difference of 2 xx 10^(6) V falls on a silver foil. The atomic number of silver is 47. Calculaye (i) the kinetic energy of the alpha -particle at the time of falling on the foil (ii) the kinetic energy of the alpha -particle at a distance of 5 xx 10^(-14) m from the nucleus and (iii) the shortest distance from the nucleus of silver to which the alpha -particle reaches.
View SolutionWhen alpha - particles are passed through a thin foil, then
View Solutionएक α− कण 2×106V के विभवांतर से गुजर कर चाँदी के एक पत्र पर आपतित होता है । चाँदी का परमाणु क्रमांक 47 है । नाभिक से 5×10−14m की दूरी पर α− कण की गतिज ऊर्जा की गणना कीजिए ।
View Solutionएक α− कण 2×106V के विभवांतर से गुजर कर चाँदी के एक पत्र पर आपतित होता है । चाँदी का परमाणु क्रमांक 47 है । α- कण की निकटतम पहुँच की दूरी ज्ञात कीजिए ।
View SolutionAn alpha -particle is accelerated through a potential of 1MV and falls on a silver foil (Z=47). Calculate : (i)Kinetic energy of the alpha -particle when it falls on the foil . (ii)Kinetic energy of the alpha -particle when it is 6.9xx10^(-14) m distant from the nucleus . (iii)Distance of closest approach to the nucleus .
View SolutionIn Rutherford experiment , the number of the alpha particles scattered through and angle of 60∘ by a silver foil is 200 per minute. When the silver foil is replaced by a copper foil of the same thickness , the number of α - particles scatted through an angle of 60∘ per minute is :
View Solution
