Which of the following acid has highest tendency to ionise in aq. Solution?
HCOOH
CH3COOH
FCH2COOH
BrCH2COOH
The correct Answer is:3
−I group increases acidity.
Topper's Solved these Questions
- TEST PAPERSBook:RESONANCE ENGLISHChapter:TEST PAPERSExercise:FST-3
Explore 30 Videos
- TEST PAPERSBook:RESONANCE ENGLISHChapter:TEST PAPERSExercise:FST 01
Explore 30 Videos
- SURFACE CHEMISTRYBook:RESONANCE ENGLISHChapter:SURFACE CHEMISTRYExercise:Section - 5
Explore 1 Video
- TEST SERIESBook:RESONANCE ENGLISHChapter:TEST SERIESExercise:CHEMISTRY
Explore 50 Videos
Similar Questions
Ultraviolet light of 5.2eV falls on Aluminium surface (work function = 3.2eV). The kinetic energy in joules of the fastest electron emitted is approximately:
View SolutionA sodium street light gives off yellow light that has a wavelength of 600nm. Then .
View SolutionMatch list I with list II and select the correct answer :
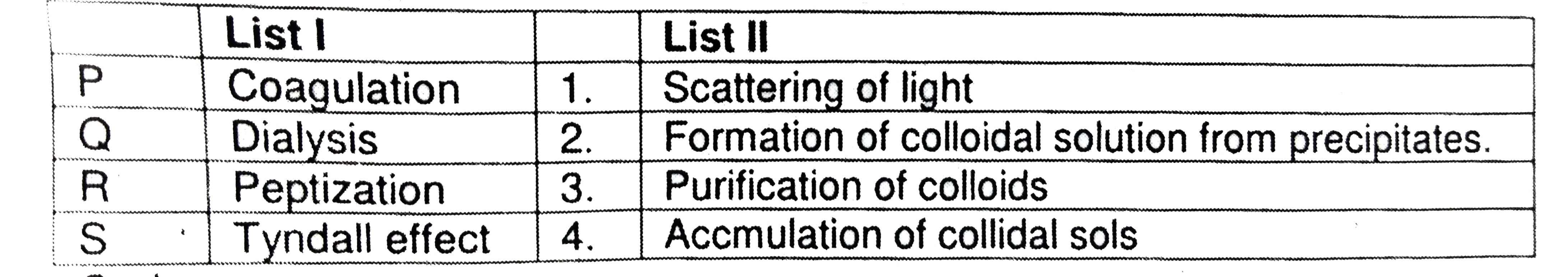
View Solutionwhich of the following plots will obtained for a conductometric titration of strong acid against a weak base?
View SolutionWhich of the following element has the highest ionisation enregy ?
View SolutionWhich of the following has the highest first ionisation energy?
View SolutionWhich of the following has highest tendency to ionise in aqueous solution ?
View SolutionWhich of the following has the highest tendency to give the reaction,
M+(g)aqueous−−−−→mediumM+(aq)?
View SolutionWhich of the following has highest ionisation energy ?
View SolutionWhich among the following has the highest ionisation energy.
View SolutionWhich of the following has the highest tendency to give the reaction M+(g)Aqueous−−−−→MediumM+(aq)
View SolutionWhich of the following has the highest normality ? ( consider each of the acid is 100% ionised )
View SolutionWhich of the following has the highest tendency to give the reaction M+(g)Aqueous−−−−→MediumM+(aq)
View SolutionWhich of the following has the highest tendency to give the reaction,
M+(g)Aqueous−−−−→MediumM+(aq)
View SolutionWhich of the following has highest first ionisation energy?
View SolutionWhich of the following has highest first ionisation energy?
View Solution
