When a liquid that is immiscible with water was steam distilled at 95.2∘C at a total pressure of 748 torr, the distilled contained 1.25 g of the liquid per gram of water . The vapour pressure of water is 648 torr at 95.2∘C, what is the molar mass of liquid?
7.975g/mol
166g/mol
145.8g/mol
None of these
The correct Answer is:A, B, C, D
For two immischible liquid ,
P∘A=Ptotal−P∘H2O=748−648⇒100
WAWB=P∘AMAP∘BMB,MA=1.251×648×18100⇒145.8
Topper's Solved these Questions
- SOLUTIONSBook:RESONANCE ENGLISHChapter:SOLUTIONSExercise:EXERCISE-1(PART-3)
Explore 3 Videos
- SOLUTIONSBook:RESONANCE ENGLISHChapter:SOLUTIONSExercise:EXERCISE-2(PART-1)
Explore 25 Videos
- SOLUTIONSBook:RESONANCE ENGLISHChapter:SOLUTIONSExercise:EXERCISE-1(PART-1)
Explore 33 Videos
- SOLUTION AND COLLIGATIVE PROPERTIESBook:RESONANCE ENGLISHChapter:SOLUTION AND COLLIGATIVE PROPERTIESExercise:PHYSICAL CHEMITRY (SOLUTION & COLLIGATIVE PROPERTIES)
Explore 51 Videos
- STEREOISOMERISMBook:RESONANCE ENGLISHChapter:STEREOISOMERISMExercise:EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))
Explore 23 Videos
Similar Questions
Statement-1 : The difference in the boiling points of equimolar solution of HCl and HF decreases as their molarity is decreased.
Statement-2 : The extent of dissociation decreases steadily with increasing dilution.
View SolutionWhat volume of 98% sulphuric acid should be mixed with water to obtain 200mL of 15% solution of sulphuric acid by weight ? Given density of H2O=1.00gcm−3, sulphuric acid (98%)=1.88gcm−3 and sulphuric acied (15%)=1.12gcm−3.
View SolutionCalculate the normality of NaOH when 2g is present in 800mL solution.
View SolutionA system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vapourizes to form a more disordered gas. When a solute is present, there is additional contribution to the entropy of the liquid due to increased randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas. Thus, a solute (non-volatile) lowers the vapour pressure of a liquid, and hence a higher boiling point of the solution.
Similarly, the greater randomness of the solution opposes the tendercy to freeze. In consequence, a lower temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution. The elevation in boiling point (ΔTb) and depression in freezing point (ΔTf) of a solution are the colligative properties which depend only on the concentration of particles of the solute and not their identity. For dilute solutions, (ΔTb) and (ΔTf) are proportional to the molarity of the solute in the solution.
To aqueous solution of Nal, increasing amounts of solid Hgl2 is added. The vapour pressure of the solution
View Solution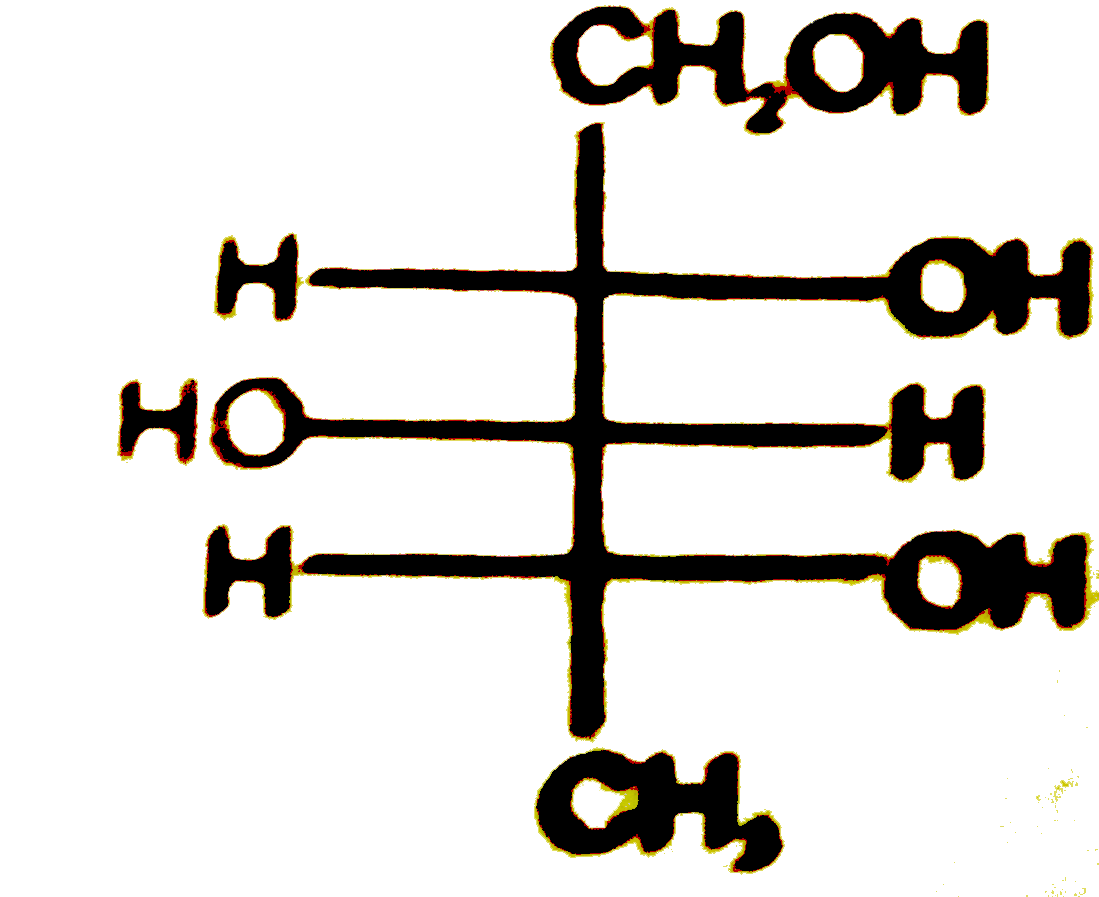 is a Fischer projection of one of_______ stereoisomers ?
is a Fischer projection of one of_______ stereoisomers ?
View SolutionA mixture of an organic liquid A and water distilled under one atmospheric pressure at 99.2^(@)C . How many grams of steam will be condensed to obtain 1.0 g of liquid A in the distillate? (Vapour pressure of water 99.2^(@)C is 739 mm Hg . Molecular weight of A=123 )
View SolutionWhen a liquid that is immiscible with water was steam distilled at 952^(@)C at a total pressure of 748 torr, the distillate contained 1.25g of the liquid per gram of water. The vapour pressure of water is 648 torr at 95.2^(@)C . What is the molar mass of liquid?
View SolutionA container contains air above liquid water. Total pressure was 800 torr. What will be the final pressure if volume is doubled ? (Aqueous tension = 40 torr).
View SolutionA liquid system consists of two immiscible liquids water and n-butyl chloride distills at one atmosphere pressure. If vapour pressuse of water at that temperature is 570 mm of Hg, then the ratio of masses of water and n-butyl chloride distilled is :
View SolutionWhen a liquid that is immiscible with water was steam distilled at 952∘C at a total pressure of 748 torr, the distillate contained 1.25g of the liquid per gram of water. The vapour pressure of water is 648 torr at 95.2∘C. What is the molar mass of liquid?
View SolutionIn the case of immiscible liquids, the addition of one liquid to another does not after the properties of either liquid. Hence liquid exerts its own vapour pressure independently of the pressure of the other. Since boiling point of any system is the temperature at which its total vapour pressure becomes equal to the prevailing pressure, so by knowing the miscibility of two liquids, we can find out boiling temperature.
An immiscible mixture of water and quinoline is prepared and distillation is done. If p∘quinoline=7.9 torr and p∘water=732.04 torr at the boiling point of 98.9∘C. Find out the relative masses of water and quinoline in the distillate. If it is given that no of moles of any component in the vapour phase ∝ its vapour pressure. (Molar mass of water = 18 g/mol and molar mass of quinoline =129g/mol)
View Solutionजल में अमिश्रणीय तथा भाप में वाष्पशील द्रवों का शोधन ………………… आसवन द्वारा किया जाता है।
View Solution100g of liquid A(molar mass 140g mol−1) was dissolved in 1000g of liquid B(molar mass 180g mol−1). The vapour pressure of pure liquid B was found to be 500 torr. Calculate the vapour pressure of pure liquid A and its vapour pressure in the solution if the total vapour pressure of the solution is 475 torr.
View SolutionA liquid is immiscible in water was steam distilled at 95.2^(@)C at a pressure of 0.983 atm. What is the mass of the liquid present per gram of water in the distullate. Molar mass of the liquid is 134.3 g/mol and the vapour pressure of water is 0.84 atm. Also, Vapour pressure of pure liquid is 0.143 atm.
View Solutionएक द्रव जो जल में अमिश्रणीय है का भाप आसवन 96∘C पर तथा कुल दाब 99.652 किलो पास्कल पर किया गया। आसुत में जल के प्रत्येक ग्राम के साथ द्रव का 1.27 ग्राम उपस्थित है। यदि जल का वाष्पदाब 96.2∘C पर 85.140 किलो पास्कल है, द्रव का मोलर द्रव्यमान क्या होगा?
View SolutionVapour pressure of a pure liquid A is 10.0 torr at 27^(@)C. The vapour pressure is lowered to 9.0 torr on dissolving one gram of B in 20g of A. If the molar mass of A is 200 g mol^(-1) then calculate the molar mass of B.
View Solution
