According to Henry' s law, the solubility of gas in a given volume of liquid increases with increase in :
temperature
Pressure
Both (A) and (B)
None of these
The correct Answer is:B
Step by step video solution for According to Henry' s law, the solubility of gas in a given volume of liquid increases with increase in : by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- SOLUTIONSBook:RESONANCE ENGLISHChapter:SOLUTIONSExercise:EXERCISE-2(PART-4)
Explore 4 Videos
- SOLUTIONSBook:RESONANCE ENGLISHChapter:SOLUTIONSExercise:EXERCISE-3(PART-1)
Explore 14 Videos
- SOLUTIONSBook:RESONANCE ENGLISHChapter:SOLUTIONSExercise:EXERCISE-2(PART-1)
Explore 25 Videos
- SOLUTION AND COLLIGATIVE PROPERTIESBook:RESONANCE ENGLISHChapter:SOLUTION AND COLLIGATIVE PROPERTIESExercise:PHYSICAL CHEMITRY (SOLUTION & COLLIGATIVE PROPERTIES)
Explore 51 Videos
- STEREOISOMERISMBook:RESONANCE ENGLISHChapter:STEREOISOMERISMExercise:EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))
Explore 23 Videos
Similar Questions
Answer the question (given below) which are based on the following diagra.
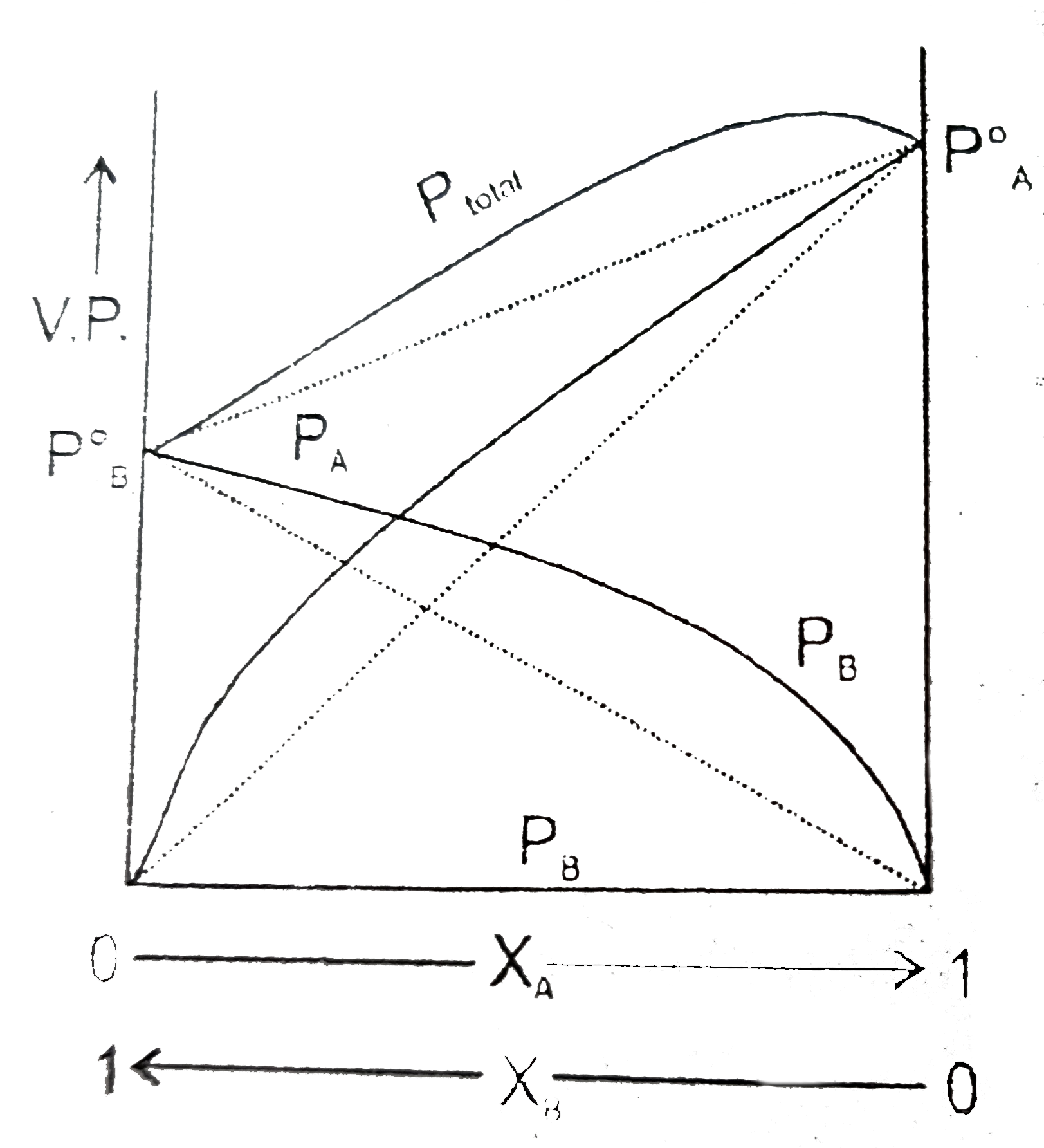
Consider some facts about the above phase diagram :
Vapour pressure diagram for real solutions of two liquids A and B that exhibit a positive deviation from Raoult's law. The vapour pressure of both A and B are greater than predicted by Raoult's law. The dashed lines lines represented the plots for ideal solutions.
A: This is observed when A...B attraction are greater than average of A...B and B...B attraction:
B: ΔHmix=+ve,ΔVmix=+ve
C: Boiling point is smaller than expected such that vaporisation is increased
D: Mixture is called azeotropic mixture
Select correct facts
View SolutionIn the depression of freezing point experimet, it is found that:
I. The vapour pressure of the solution is less than that of pure solvent.
II. The vapour pressure of the solution is more than that of pure solvent.
III. Only solute molecules solidify at the freezing point.
IV. Only solvent molecules solidify at the freezing point.
View SolutionWhat volume of 98% sulphuric acid should be mixed with water to obtain 200mL of 15% solution of sulphuric acid by weight ? Given density of H2O=1.00gcm−3, sulphuric acid (98%)=1.88gcm−3 and sulphuric acied (15%)=1.12gcm−3.
View SolutionA system of greater disorder of molecules is more probable. The disorder of molecules is reflected by the entropy of the system. A liquid vapourizes to form a more disordered gas. When a solute is present, there is additional contribution to the entropy of the liquid due to increased randomness. As the entropy of solution is higher than that of pure liquid, there is weaker tendency to form the gas. Thus, a solute (non-volatile) lowers the vapour pressure of a liquid, and hence a higher boiling point of the solution.
Similarly, the greater randomness of the solution opposes the tendercy to freeze. In consequence, a lower temperature must be reached for achieving the equilibrium between the solid (frozen solvent) and the solution. The elevation in boiling point (ΔTb) and depression in freezing point (ΔTf) of a solution are the colligative properties which depend only on the concentration of particles of the solute and not their identity. For dilute solutions, (ΔTb) and (ΔTf) are proportional to the molarity of the solute in the solution.
To aqueous solution of Nal, increasing amounts of solid Hgl2 is added. The vapour pressure of the solution
View Solution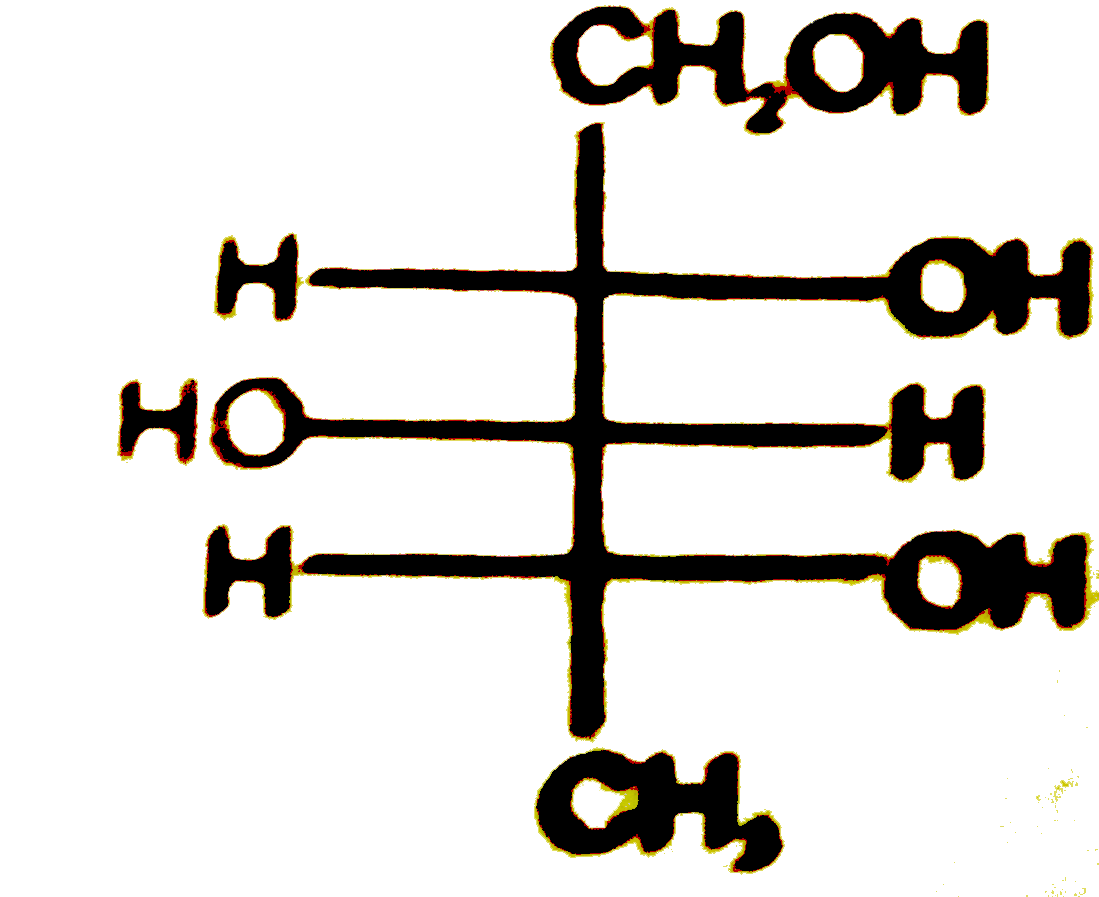 is a Fischer projection of one of_______ stereoisomers ?
is a Fischer projection of one of_______ stereoisomers ?
View SolutionSolubility of a gas in liquid increases on
View SolutionSolubility of a gas in liquid increases on:
View SolutionAccording to William Henry,the solubility of a gas in liquid depends on the pressure of the gas .if 'm' is the molality of the gas and'P' is its pressure then which of the following plot is in accordance with the law:
View SolutionAccording to Henry's law, the solubility of a gas in a given volume of liquid increases with increases in :
View SolutionThe solubility of a gas in liquid increases with
View SolutionSolubility of a gas in a liquid increases with
View SolutionAn increase in ............... increases the solubility of a gas in liquid.
View SolutionThe solubility of gas in liquid increases with
View SolutionAccording to Henry's law, the solubility of a gas in a given volume of liquid increases with increases in :
View SolutionThe solubility of a gas in liquid increases with
View Solutionकिसी द्रव में गैस की विलेयता में वृद्धि किस प्रकार की जा सकती है?
View Solution
