An aqueous solution of glucose boils at 100.10∘C.The molal elevation constant for water is 0.5kmol−1kg. The number of molecules of glucose in the solution containing 100g of water is
1.18 x 1021
1.20 x 1022
7.08 x 1022
6.08 x 1021
The correct Answer is:C
NA
Topper's Solved these Questions
- TEST PAPERSBOOK - RESONANCE ENGLISHCHAPTER - TEST PAPERSEXERCISE - PART - III CHEMISTRY SEC - 1
12 Videos
- TEST PAPERSBOOK - RESONANCE ENGLISHCHAPTER - TEST PAPERSEXERCISE - PART - III CHEMISTRY SEC - 2
18 Videos
- SURFACE CHEMISTRYBOOK - RESONANCE ENGLISHCHAPTER - SURFACE CHEMISTRYEXERCISE - Section - 5
1 Video
- TEST SERIESBOOK - RESONANCE ENGLISHCHAPTER - TEST SERIESEXERCISE - CHEMISTRY
50 Videos
Similar Questions
10dm3 of N2 gas and 10dm3 of gas X contain the same number of molecules at the same temperature, the gas (X) may be
View SolutionWhat is power (in watt) of a light source emitting 3.125×1018 photons per second, while the wavelength of light is 620nm ? (use hc=12400eVÅ)
View SolutionMatch list I with list II and select the correct answer :
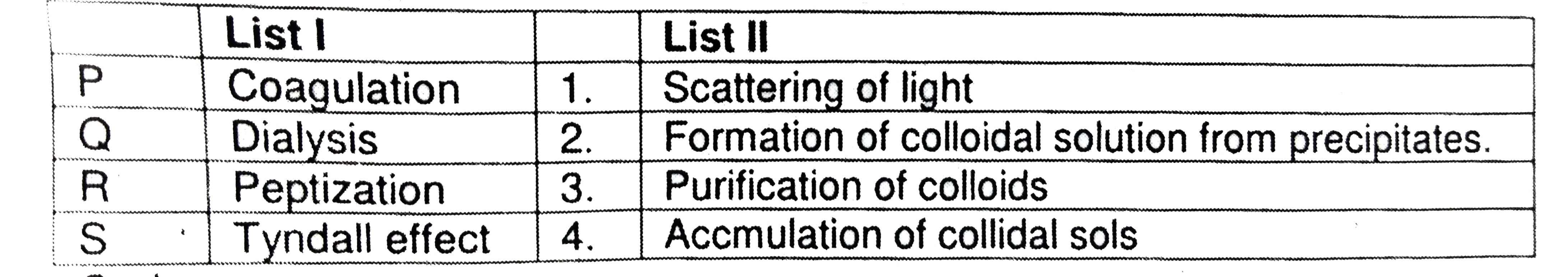
View Solutionwhich of the following plots will obtained for a conductometric titration of strong acid against a weak base?
View SolutionAn aqueous solution of glucose boils at 100.01∘C.The molal elevation constant for water is 0.5kmol−1kg. The number of molecules of glucose in the solution containing 100g of water is
View SolutionThe rise in the boiling point of a solution containing 1.8 g of glucose in 100 g of solvent is 0.1∘C. The molal elevation constant of the liquid is
View Solutionग्लूकोज के एक जलीय विलयन जिसमे 12 g ग्लूकोज (M=180)100g जल में घुला है, का क्वथनांक 100.34∘Cहै तथा जल का क्वथनांक 100∘C है. जल के मोलल उन्नयन स्थिराक की गणना कीजिए.
View Solution0.1 molal aqueous solution of glucose boils at 100.16∘C . The boiling point of 0.5 molal aqueous solution of glucose will be ______.
View SolutionAn aqueous solution of glucose boils at 100.01^(@)C . The molal elevation constant for water is 0.5 K kg mol^(-1) . What is the number of glucose molecules in the solution containing 100 g of water ?
View SolutionAn aqueous solution of glucose containing 12g in 100g of water was found to boil at 100.34∘C.
Calculate of Kb for water in Kmol−1Kg.
View SolutionThe boiling point of aqueous solution of glucose is 100.01 C What is the number of glucose molecules in 100g water of this solution? [ k_b = 0.5K kg mol^-1 ]
View Solution0.1 molal aqueous solution of glucose boils at 100.16 ""^(@)C . The boiling point of 0.5 molal aqueous solution of glucose will be ______.
View SolutionThe boiling point of pure water is 373 K. calculate the boiling point of an aqueous solution containing 18 g of glucose (M.W. = 180) in 100g of water. Molal elevation constant of water is 0.52 K kg mol−1.
View SolutionAn aqueous solution of glucose boils at 100.01^(@)C .The molal elevation constant for water is 0.5 kmol^(-1)kg . The number of molecules of glucose in the solution containing 100g of water is
View SolutionAn aqueous solution of glucose boils at 100.02∘C.The molal elevation constant for water is 0.5kmol−1kg. The number of molecules of glucose in the solution containing 100g of water is
View SolutionAn aqueous solution of glucose boils at 100.03^(@)C .The molal elevation constant for water is 0.5 kmol^(-1)kg . The number of molecules of glucose in the solution containing 100g of water is
View Solution

