The substance undergoes first order decomposition.The decomposition follows two parallel first order reactons as :
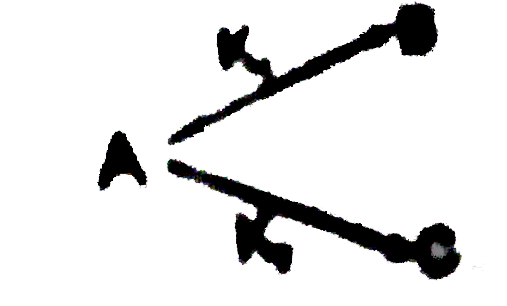
K1=10−2sec−1andK2=4⋅10−2sec−1
If the corresponding activation energies of parallel reaction are 100 and 120 kJ mol−1 then the net activation energy of A is/are:
120 kJ mol−1
116 kJ mol−1
100 kJ mol−1
150 kJ mol−1
The correct Answer is:B
E=k1E1+k2E2k1+k2=10−2×100+120×4×10−210−2+4×10−2=116kJmol−1
टॉपर्स ने हल किए ये सवाल
समान प्रश्न
A red solide is insoluble in water. However it becomes soluble if some KI is added to water. On heating the red solid in a test tube, there is liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The red solid is :
View SolutionSodium (Na =23) crystallizen in bcc arrangement with the interfacial separation between the atoms at the edge 53.6pm. The density of sodium crystal is:
View SolutionA substance undergoes first order decomposition. The decomposition follows two parallel first order reaction as: and KP_(1)=1.26xx10^(-4)sec^(-1) K_(2)=3.80xx10^(-5)sec^(-1) The percentage distribution of B and C are:
View SolutionConsider the reaction.
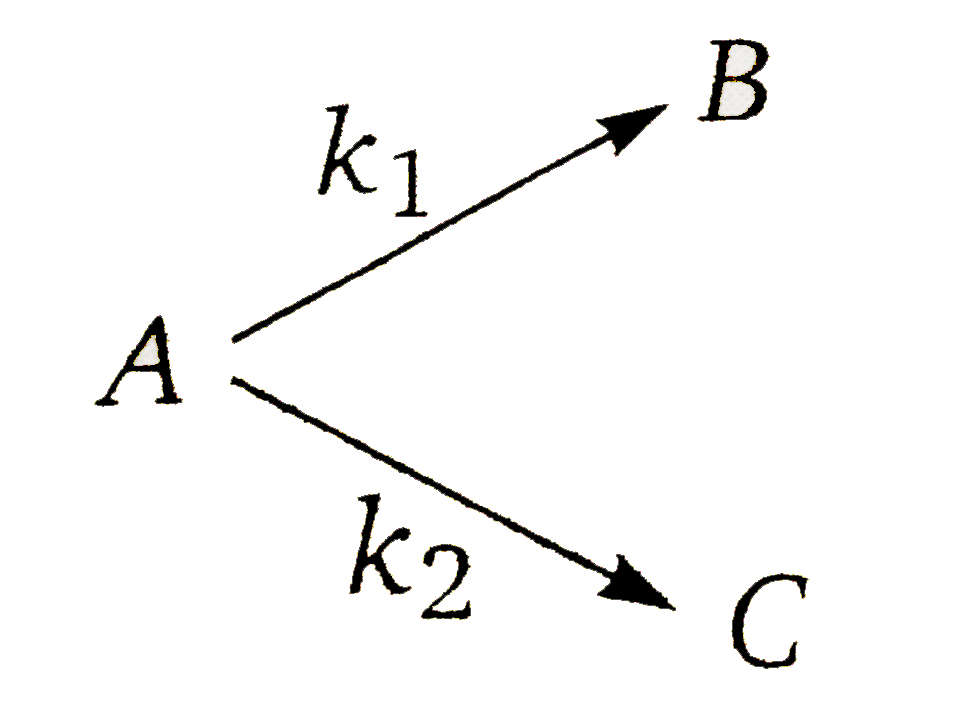
The rate constant for two parallel reactions were found to be 10−2dm−2mol−1s−1 and 4×10−2dm−3mol−1s−1 . If the corresponding energies of activation of the parallel reaction are 100 and 120 kJ/mol respectively, what is the net energy of activation (Ea) of A
View SolutionThe substance undergoes first order decomposition.The decomposition follows two parallel first order reactons as : K_1=10^(-2) sec^(-1) and K_2=10^(-2)sec^(-1) If the corresponding activation energies of parallel reaction are 100 and 120 kJ "mol"^(-1) then the net activation energy of A is/are:
View SolutionA substance undergoes first order decomposition. The decomposition follows two parallel first order reaction as :
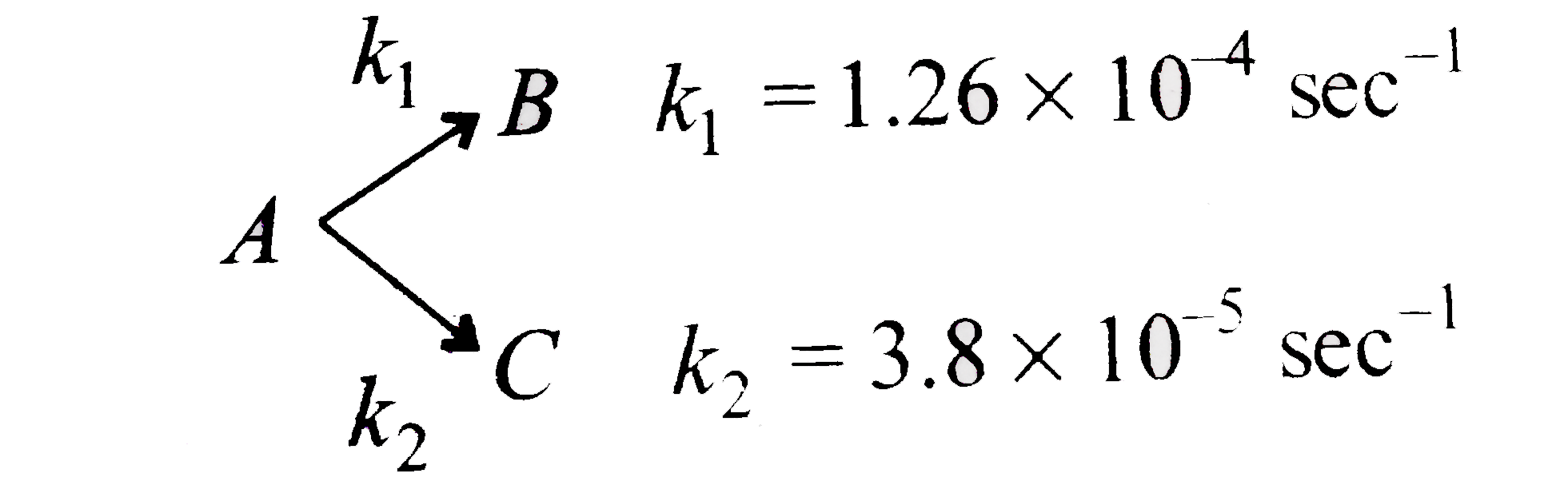
The precentage distribution of B and C are :
View SolutionA substance undergoes first order decomposition. The decomposition follows two parallel first order reactions as :
k1=1.26×10−4s−1,k2=3.8×10−5s−1
The percentage distribution of B and C are :
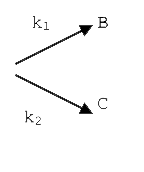
View SolutionThe substance undergoes first order decomposition. The decomposition follows two parallel first order reactions as : K_(1)=1.26xx10^(-4) sec^(-1) and K_(2) =3.8xx10^(-5) sec^(-1) The percentage distribution of B and C
View SolutionThe energy of activation and specific rate constant for a first order reaction at 25^(@)C are 100 kJ/mole and 3.46 xx 10^(–5)" sec"^(–1) respectively. Determine the temperature at which half life of the reaction is 2 hours.
View SolutionA substance undergoes first order decomposition. The decomposition follows two parallel first order reactions as , k_(1)=1.26xx10^(-4)" sec"^(-1) and k_(2)=3.6xx10^(-5)" sec"^(-1) Calculate the % distribution of B & C.
View SolutionThe substance undergoes first order decomposition. The decomposition follows two parallel first order reactions as :
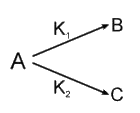
K1=1.26×10−4sec−1andK2=3.8×10−5sec−1 The percentage distribution of B and C
View SolutionA substance undergoes a first order decomposition. The decomposition follows two parallel first order reactions as
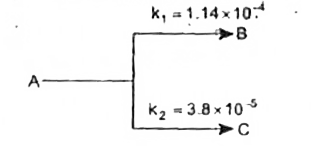
The percentage distribution of B and C are
View SolutionA substance undergoes first order decomposition. The decomposition follows two parallel first order reactios as: and K_(1)=1.26xx10^(-4)"sec"^(-1),K_(2)=3.80xx10^(-5)"sec"^(-1) The percentage distribution of B and C are
View SolutionConsider the reaction. The rate constant for two parallel reactions were found to be 10^(-2) dm^(-2)mol^(-1)s^(-1) and 4xx10^(-2)dm^(-3)mol^(-1)s^(-1) . If the corresponding energies of activation of the parallel reaction are 100 and 120 kJ/mol respectively, what is the net energy of activation (E_(a)) of A
View SolutionDecomposition of A follows two parallel 1st order reactions with K1=1.3×10−4sec−1 and K2=3.9×10−5sec−1. Find % distribution of the products.
View Solution

