For a hypotherical elementary reaction 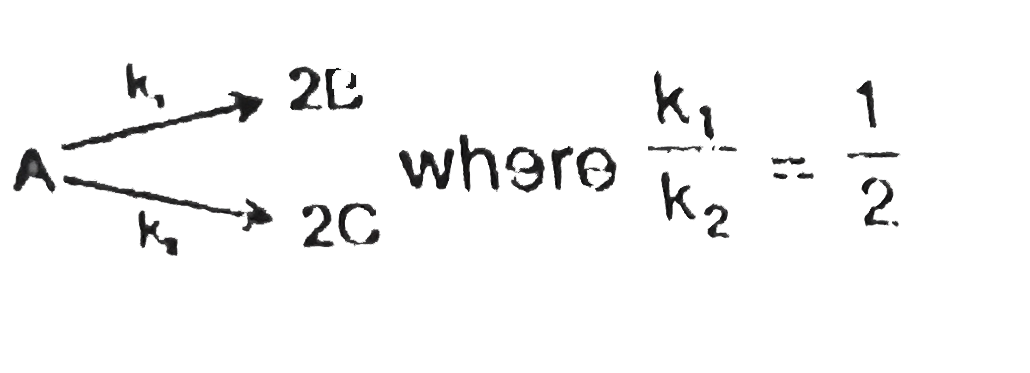 where k1k2=12
where k1k2=12
Initailly only 2 moles of A are present.
Number of moles of B are , if reactions gets 50 % completed :
2
1
0.666
0.333
The correct Answer is:C
Mole of B =21+2=0.666
Topper's Solved these Questions
Similar Questions
For a hypotherical elementary reaction
 where k1k2=12
where k1k2=12
Initailly only 2 moles of A are present.
The sum of mole of (B) are ( C) at end of reaction is :
View SolutionFor a hypotherical elementary reaction
 where k1k2=12
where k1k2=12
Initailly only 2 moles of A are present.
The total number of moles A,B and C at the end of 50% reaction are :
View Solution
Knowledge Check
In the reaction A+B⇔C+D, 4 moles of A
react with 4 moles of B and form 2 moles each of C and D. The value of Ke for the reaction isA2B3C1D1.56
Rate law for the elementary reaction 2AB→k1A2+B2 will be:
View SolutionFor the reaction A+2B→C,5moles of A and 8moles of B will produce :-
View SolutionFor the reaction A+2B→C, 5 moles of A and 8 mole of B will produce
View SolutionIn an elementary reaction A+2B→products, the molecularity of the reaction is:
View SolutionA mixture of SO3,SO2 and O2 gases is maintained in a 10L flask at a temperature at which the equilibrium constant for the reaction is 100:
2SO2(g)+O2(g)⇔2SO3(g)
a. If the number of moles of SO2 and SO3 in the flask are equal. How many moles of O2 are present?
b. If the number of moles of SO3 in flask is twice the number of moles of SO2, how many moles of oxygen are present?
View SolutionFor the following elementary first order reaction:
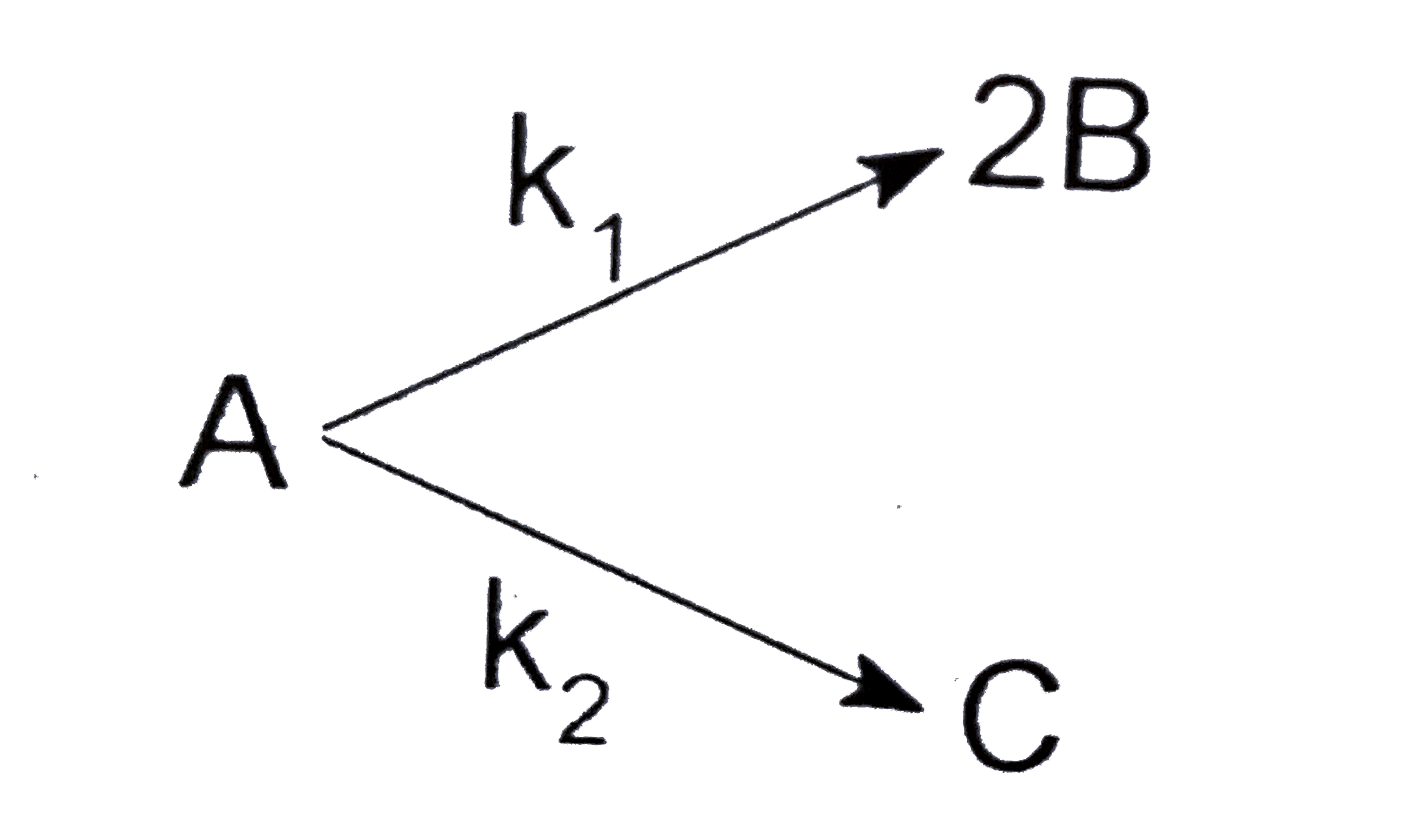
If k2=2k1, then % of B in overall product is:
View Solution9 moles of "D" and 14 moles of E are allowed to react in a closed vessel according to given reactions. Calculate number of moles of B formed in the end of reaction, if 4 moles of G are present in reaction vessel. (percentage yield of reaction is mentioned in the reaction) Step -1 3D+4E80%→5C+A Step-2 3C+5G50%→6B+F
View Solution
