Statement -1: The magnitude of the work involved in an isothermal expansion is greater than that involved in an adiabatic expansion.
Statement -2: P-v curve (P on y -axis and V on X-axis) decrease more rapidly for reversible adiabatic expansion compared to reversible isothermal expansion starting from same initial state.
Statement-1 is True, Statement-2 is True, Statement-2 is a correct explanation for Statement-1
Statement-1 is True, Statement-2 is True, Statement-2 is NOT a correct explanation for Statement-1
Statement-1 is True, Statement-2 is False.
Statement-1 is False, Statement-2 is True.
The correct Answer is:A
NA
Topper's Solved these Questions
Similar Questions
A red solide is insoluble in water. However it becomes soluble if some KI is added to water. On heating the red solid in a test tube, there is liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The red solid is :
View SolutionSodium (Na =23) crystallizen in bcc arrangement with the interfacial separation between the atoms at the edge 53.6pm. The density of sodium crystal is:
View SolutionA sample of ideal gas undergoes isothermal expansion in a reversible manner from volume V_(1) to volume V_(2) . The initial pressure is P_(1) and the final pressure is P_(2) . The same sample is then allowed to undergoes reversible expansion under adiabatic conditions from volume V_(1) to V_(2) . The initial pressure being same but final pressure is P_(2) . The work of expansion in adiabatic process (w_(adi)) is related to work of expansion in isothermal process (w_(iso)) is
View SolutionIf a certain mass of gas is made to undergo separately adiabatic and isothermal expansions to the same pressure, starting form the same initial conditions of temperature and pressure, then, as compared to that of isothermal expansion, in the case of adiabatic expansion, the final
View SolutionStatementI: Work done by a gas in isothermal expansion is more than the work done by the gas in the same expansion adaibatically.
Statement II: Temperature remains constant in isothermal ecpansion but not in adiabatic expansion.
View SolutionThe magnitude of enthalpy changes for irreversible adiabiatic expansion of a gas from 1 L to 2L is DeltaH_(1) and for reversible adiabatic expansion for the same expansion is DeltaH_(2) . Then :
View SolutionStaetement -1: The magniyude of the work involed in an isothermal expansion is greater than that involved in an adiabatic expansion. Statement -2: P-v cure (pon y -axas and V on X-axas) decrease more repidly for reversible asiabatic expansion compared to reversible isothermal expansion starting from same initial state.
View SolutionStaetement -1: The magniyude of the work involed in an isothermal expansion is greater than that involved in an adiabatic expansion.
Statement -2: P-v cure (pon y -axas and V on X-axas) decrease more repidly for reversible asiabatic expansion compared to reversible isothermal expansion starting from same initial state.
View Solutionदिए हुए रेखाचित्र में एक आदर्श गैस के लिए रूद्धोष्म (adiabatic) और समतापी (isothermal) अवस्थाओं में उत्क्रमणीय प्रसरण (reversible expansion) दिखाया गया है। निम्नलिखित प्रकथनों में से कौन-सा कथन सही है/हैं?
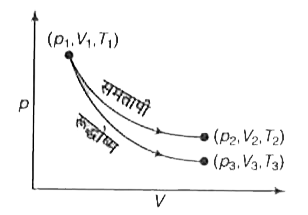
View SolutionA : Work done by a gas in isothermal expension is more than the work done by the gas in the same expasion adiabatically.
R : Temperature remains constant in isothermal expansion and not in adiabatic expansion.
View Solutionदिये हुए रेखाचित्र में एक आदर्श गैस के लिए रूद्धोष्म (adiabatic) और समतापी (isothermal) अवस्थाओं में उत्क्रमणीय प्रसार (reversible expansion) दिखाया गया है। निम्नलिखित प्रकथनों में से कौन प्रकथन सही है/ हैं
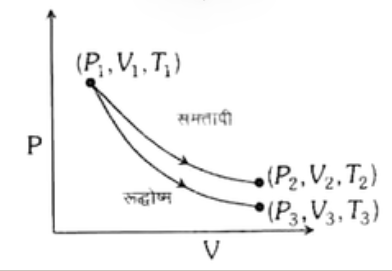
View SolutionThe magnitudes of enthalpy changes for irreversible adiabatic expansion of a gas from 1L to 2L is ΔH1 and for reversible adiabatic expansion for the same expansion is ΔH2. Then
View SolutionStaetement -1: The magniyude of the work involed in an isothermal expansion is greater than that involved in an adiabatic expansion.
Statement -2: P-v cure (pon y -axas and V on X-axas) decrease more repidly for reversible asiabatic expansion compared to reversible isothermal expansion starting from same initial state.
View SolutionOn a P-V diagram starting from an initial state (P_0 V_0) plot an adiabatic expansion to 2V_0 an isothermal expansion to 2V_0 and an isobaric expansion to 2V_0 Use this graph to determine in which process the least work is done by the system
View SolutionWork done in the isothermal reversible expansion of a gas is always greater than that done in isothermal irreversible expansion of the gas.
View SolutionThis question has Statement I and Statement II. Of the four choices given after the Statements, choose the one that best describes the two Statements. Statement-I : The magnitude of the work involved in an isothermal expansion is greater than that involved in an adiabatic expansion. Statement-II : P-V curve (P on y-axis and V on x-axis) decrease more rapidly for reversible adiabatic expansion compared to reversible isothermal expansion starting from same initial state.
View Solution
