Which of the following are the correct :
A Catalyst remains unchanged in mass and chemical compositions at the end of reactions.
Finely divided state of catalyst is more efficient for the reaction
Catalyst change equilibrium state of the reaction
A catalyst changes the entropy and the free energy of a reaction
The correct Answer is:A,B
To solve the question regarding the correctness of the statements about catalysts, we will analyze each statement one by one.
1. Statement 1: A catalyst remains unchanged in mass and chemical properties at the end of the reaction.
- Analysis: A catalyst is a substance that increases the rate of a chemical reaction without undergoing any permanent change itself. At the end of the reaction, the catalyst can be recovered in its original form, meaning its mass and chemical properties remain unchanged.
- Conclusion: This statement is correct.
2. Statement 2: The finely divided state of a catalyst is more effective for the reaction.
- Analysis: The effectiveness of a catalyst is often related to its surface area. A finely divided catalyst has a larger surface area compared to a bulk catalyst, allowing more active sites for the reaction to occur. This increases the catalytic efficiency.
- Conclusion: This statement is correct.
3. Statement 3: A catalyst changes the equilibrium state of the reaction.
- Analysis: A catalyst does not change the position of equilibrium in a chemical reaction. It only speeds up the rate at which equilibrium is reached. The concentrations of reactants and products at equilibrium remain the same regardless of the presence of a catalyst.
- Conclusion: This statement is incorrect.
4. Statement 4: A catalyst changes the entropy and the free energy of a reaction.
- Analysis: A catalyst affects the rate of a reaction but does not change the thermodynamic properties such as entropy and free energy. The overall energy change (ΔG) and entropy change (ΔS) of the reaction remain the same regardless of the presence of a catalyst.
- Conclusion: This statement is incorrect.
Final Summary:
- Correct Statements: Statement 1 and Statement 2.
- Incorrect Statements: Statement 3 and Statement 4.
- Analysis: A catalyst is a substance that increases the rate of a chemical reaction without undergoing any permanent change itself. At the end of the reaction, the catalyst can be recovered in its original form, meaning its mass and chemical properties remain unchanged.
- Conclusion: This statement is correct.
- Analysis: The effectiveness of a catalyst is often related to its surface area. A finely divided catalyst has a larger surface area compared to a bulk catalyst, allowing more active sites for the reaction to occur. This increases the catalytic efficiency.
- Conclusion: This statement is correct.
- Analysis: A catalyst does not change the position of equilibrium in a chemical reaction. It only speeds up the rate at which equilibrium is reached. The concentrations of reactants and products at equilibrium remain the same regardless of the presence of a catalyst.
- Conclusion: This statement is incorrect.
- Analysis: A catalyst affects the rate of a reaction but does not change the thermodynamic properties such as entropy and free energy. The overall energy change (ΔG) and entropy change (ΔS) of the reaction remain the same regardless of the presence of a catalyst.
- Conclusion: This statement is incorrect.
- Correct Statements: Statement 1 and Statement 2.
- Incorrect Statements: Statement 3 and Statement 4.
Topper's Solved these Questions
Similar Questions
A red solide is insoluble in water. However it becomes soluble if some KI is added to water. On heating the red solid in a test tube, there is liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The red solid is :
View SolutionSodium (Na =23) crystallizen in bcc arrangement with the interfacial separation between the atoms at the edge 53.6pm. The density of sodium crystal is:
View SolutionWhich of the following statements is/are correct about the following is/are correct about the following reactions ?
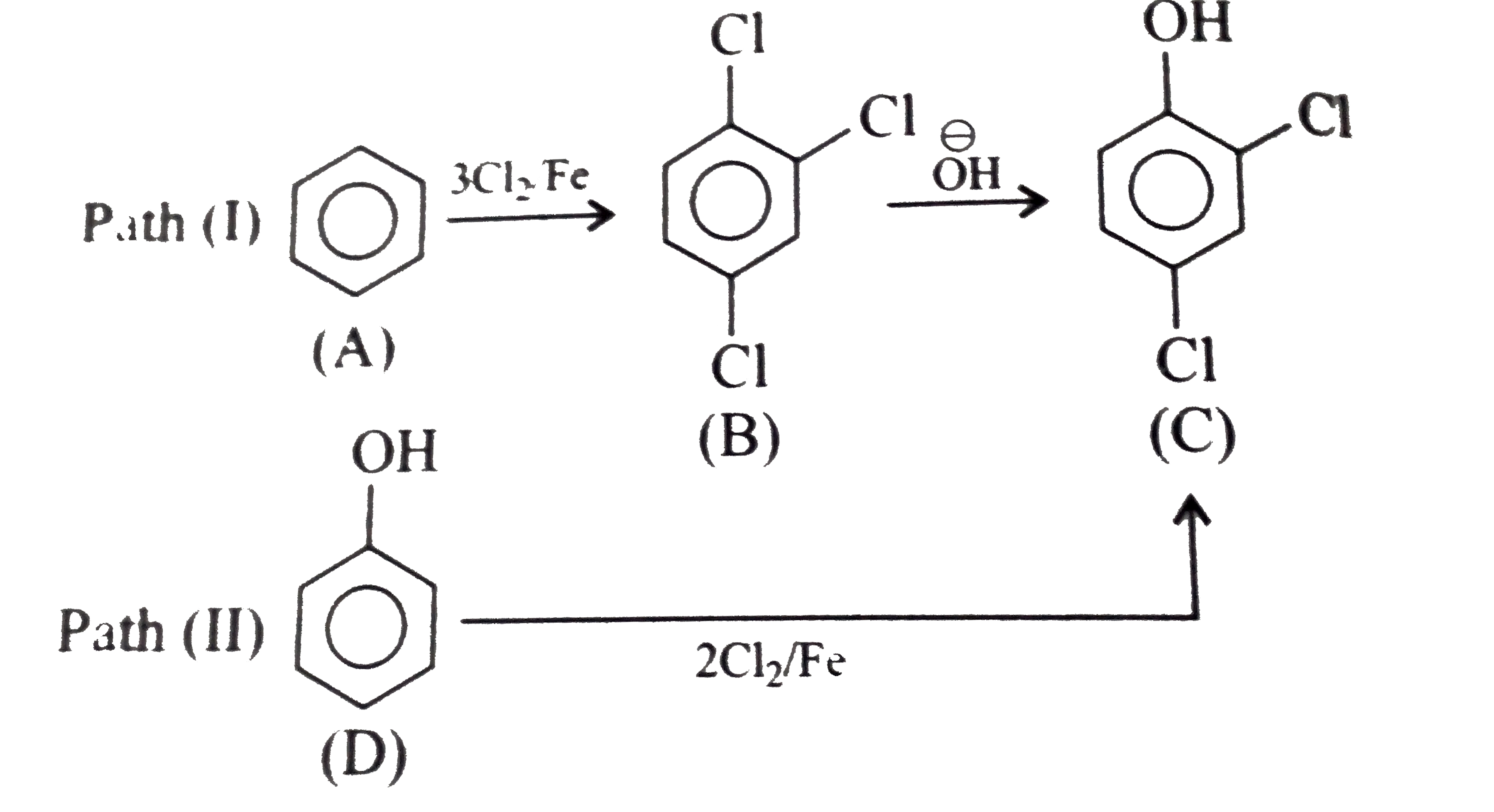
View SolutionWhich of the following are correct reactions ?
View SolutionWhich of the following is not correct ?
View SolutionWhich of the following statement is not correct ?
View SolutionWhich of the following statement is/are correct ?
View SolutionWhich of the following statement is correct ?
View Solutionगेटो के निम्न संयुग्मन के लिए कौन-सी सत्यता सारणी सही है -
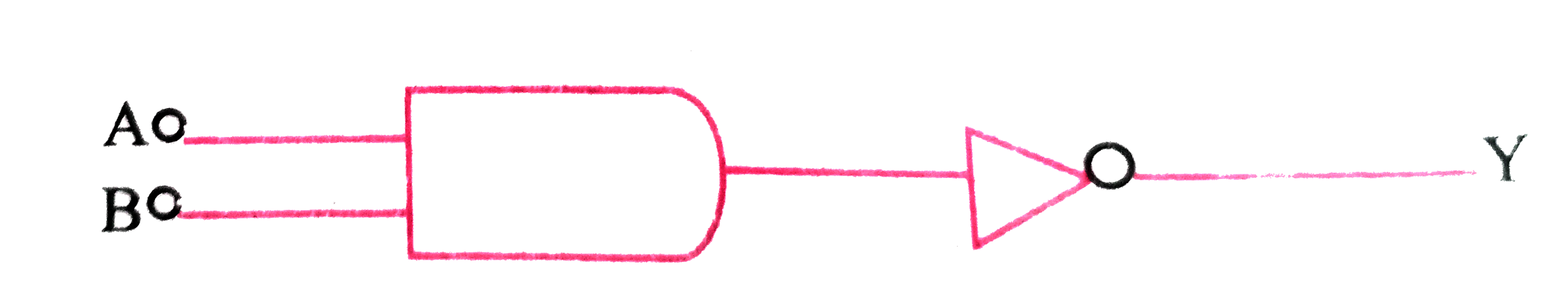
View SolutionWhich of the following is not correct?
View SolutionWhich of the following statements are correct ?
View SolutionMultiple correct postmodern Which of the following statements is correct?
View SolutionChoose the correct option.
Which is following relations is correct?
View Solutionसही उत्तर चुनें-
निम्नलिखित में कौन सही है ?
View Solutionwhich of the following is correct for the following graph?
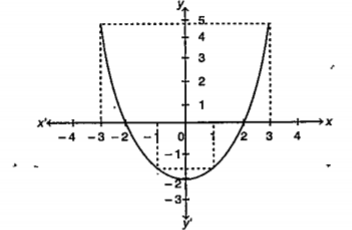
View SolutionWhich of the following statements is/are correct about the following is/are correct about the following reactions ?
View Solution

