How many maximum atoms lie in the same plane in the structure of methylenesulphurtetrafluoride (CH2SF4) ?
The correct Answer is:6
To determine how many maximum atoms lie in the same plane in the structure of methylenesulphurtetrafluoride (CH₂SF₄), we can follow these steps:
Step 1: Understand the Molecular Structure
Methylene sulfur tetrafluoride consists of a sulfur atom (S) bonded to a carbon atom (C), which is further bonded to two hydrogen atoms (H) and four fluorine atoms (F).
Step 2: Draw the Lewis Structure
1. Start by placing the sulfur atom at the center.
2. Attach the carbon atom to the sulfur with a double bond.
3. Attach two hydrogen atoms to the carbon atom.
4. Attach four fluorine atoms to the sulfur atom, with two in the equatorial position and two in the axial position.
Step 3: Identify the Plane of Atoms
In the structure of CH₂SF₄:
- The carbon atom (C) and the two hydrogen atoms (H) are in the same plane.
- The two fluorine atoms (F) in the equatorial position also lie in this plane.
Step 4: Count the Atoms in the Same Plane
Now, we can count the total number of atoms in the same plane:
- 1 Carbon (C)
- 2 Hydrogens (H)
- 1 Sulfur (S)
- 2 Fluorines (F)
Adding these together gives:
1 (C) + 2 (H) + 1 (S) + 2 (F) = 6 atoms.
Conclusion
The maximum number of atoms that lie in the same plane in the structure of methylenesulphurtetrafluoride (CH₂SF₄) is 6.
---
Methylene sulfur tetrafluoride consists of a sulfur atom (S) bonded to a carbon atom (C), which is further bonded to two hydrogen atoms (H) and four fluorine atoms (F).
1. Start by placing the sulfur atom at the center.
2. Attach the carbon atom to the sulfur with a double bond.
3. Attach two hydrogen atoms to the carbon atom.
4. Attach four fluorine atoms to the sulfur atom, with two in the equatorial position and two in the axial position.
In the structure of CH₂SF₄:
- The carbon atom (C) and the two hydrogen atoms (H) are in the same plane.
- The two fluorine atoms (F) in the equatorial position also lie in this plane.
Now, we can count the total number of atoms in the same plane:
- 1 Carbon (C)
- 2 Hydrogens (H)
- 1 Sulfur (S)
- 2 Fluorines (F)
1 (C) + 2 (H) + 1 (S) + 2 (F) = 6 atoms.
The maximum number of atoms that lie in the same plane in the structure of methylenesulphurtetrafluoride (CH₂SF₄) is 6.
Topper's Solved these Questions
Similar Questions
A red solide is insoluble in water. However it becomes soluble if some KI is added to water. On heating the red solid in a test tube, there is liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The red solid is :
View SolutionSodium (Na =23) crystallizen in bcc arrangement with the interfacial separation between the atoms at the edge 53.6pm. The density of sodium crystal is:
View SolutionHow many maximum atom (s)is//are are present in same plane of Cr(CO)_(6) ? .
View SolutionHow many carbon atoms lie in a plane in [Ni(dmg)2]?
View SolutionIn which of the following structures the pair of starred carbon atoms do not lie in the same plane?
View SolutionHow many maximum atoms lie in the same plane in the structure of methylenesulphurtetrafluoride (CH2SF4) ?
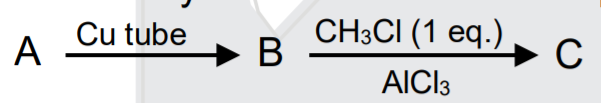
View SolutionHow many atoms lie in the same plane in the major product (C) ? (Where A is the alkyne of lowest molecular mass)
View SolutionIn which of the following species maximum atom can lie in same plane ?
View SolutionIn which of the following species maximum atom can lie in same plane ?
View SolutionIn which of the following species maximum atom can lie in same plane ?
View SolutionHow many atoms lie in the same plane in the major product (C) ? (Where A is the alkyne of lowest molecular mass)
View SolutionHow many atoms lie in the same plane in the major product (C) ? (Where A is the alkyne of lowest molecular mass)

View SolutionBF3 में कितने परमाणु समान तल में है |
View SolutionPCl5 में अधिकतम कितने परमाणु समान तल में है।
View Solutionबेंजीन में अधिकतम कितने परमाणु समान तल में होते है ?
View Solutionनिम्न अणु में कितने परमाणु समान तल में है ?
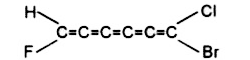
View Solution
