A crystalline solid (A) reacts with hydrogen gas to form a monoatomic gas (B) and a highly associated liquid ( C).(A) is soluble in water and undergoes hydroloysis slowly to form (B),(C ) and a diatomic gas (D).When pure and dry (D) is subjected to a silent electric discharge an another pale blue gas (E) is produced which like (A) also acts a strong oxidising agent.(E) dissolves in potassium hydroxide forming an orange coloured solid which is paramagnetic in nature. (A) reacts with IF5,SbF5,PF5 etc to form the addition compounds. (C ) forms an addition compound (F) with KF with when electrolysed in molten state forms a most reactive gas (G).(B) and (G) (taken in 2:1) ratio when heated in nickel tube at 400∘C and 6 atm pressure forms (A).The first ionization energy (IE1) of (B) is fairly close to (D).
Which of the following statements is correct for (A) ?
The central atom of (A) is sp3d hybridised with three lone pairs of electrons
(A) reacts with S8 molecules in presence of (C ) to form a compound in which the S atom is sp3d2 hybridised
(A) reacts with SbF5 as a lewis base
All of these
The correct Answer is:D
(A)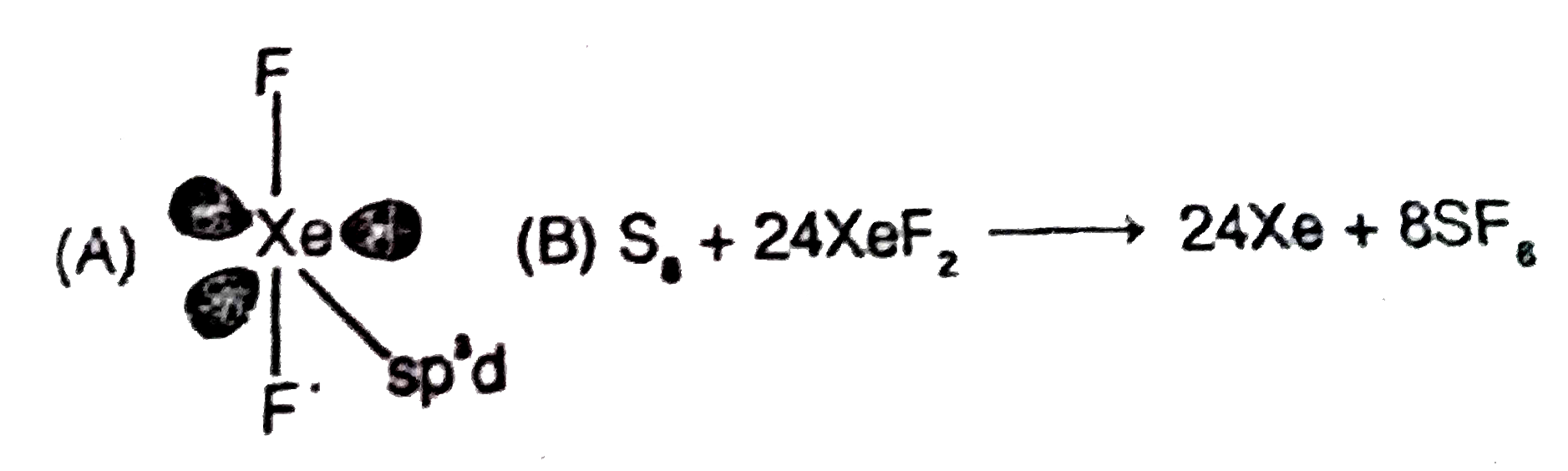
(B)S8+24XeF2→24Xe+8SF6(S→sp3d2)
(C )XeF2+SbF5→[XeF]+[SbF6]−
Topper's Solved these Questions
Similar Questions
A red solide is insoluble in water. However it becomes soluble if some KI is added to water. On heating the red solid in a test tube, there is liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The red solid is :
View SolutionSodium (Na =23) crystallizen in bcc arrangement with the interfacial separation between the atoms at the edge 53.6pm. The density of sodium crystal is:
View SolutionAn orange solid (A) on heating gave a green residue (B), colourless gas (C) and water vapour. The dry gas (C) on passing over heated magnesium gave a white solid (D).(D) on reaction with water have a gas (E) which formed dense white fumes with HCl. Indentify (A) to (E) and give the reactions.
View SolutionAn orange solid a on heating gave a green residue B, a colourless gas c and water vapour. The dry gas c on passing over heated Mg gave a white solid D. D on reaction with water gave a gas E which formed dense white fumes with HCl. Identify A to E and give the reaction involved.
View SolutionWhite crystalline solid (A) reacts with H2 to form a highly associated liquid (B) and a monoatomic , colourless gas (C ). The liquid (B) is used for etching glass. Compound (A) undergoes hydrolysis slowly to form (C ), (B) and a diatomic gas (D ) whose IE is almost similar to that of (C ). (B) forms an addition compound with KF to form (E) which is electrolysed in the molten state to form a most reactive gas (F) which combines with (C ) in 2:1 ratio of produce (A).
Which of the following is correct for the white crystalline solid (A) ?
View SolutionWhite crystalline solid (A) reacts with H2 to form a highly associated liquid (B) and a monoatomic , colourless gas (C ). The liquid (B) is used for etching glass. Compound (A) undergoes hydrolysis slowly to form (C ), (B) and a diatomic gas (D ) whose IE is almost similar to that of (C ). (B) forms an addition compound with KF to form (E) which is electrolysed in the molten state to form a most reactive gas (F) which combines with (C ) in 2:1 ratio of produce (A).
According to Molecular Orbital Theory, which of the following is correct about the molecule (D) ?
View SolutionA crystalline solid (A) reacts with hydrogen gas to form a monoatomic gas (B) and a highly associated liquid ( C).(A) is soluble in water and undergoes hydroloysis slowly to form (B),(C ) and a diatomic gas (D).When pure and dry (D) is subjected to a silent electric discharge an another pale blue gas (E) is produced which like (A) also acts a strong oxidising agent.(E) dissolves in potassium hydroxide forming an orange coloured solid which is paramagnetic in nature. (A) reacts with IF5,SbF5,PF5 etc to form the addition compounds. (C ) forms an addition compound (F) with KF with when electrolysed in molten state forms a most reactive gas (G).(B) and (G) (taken in 2:1) ratio when heated in nickel tube at 400∘C and 6 atm pressure forms (A).The first ionization energy (IE1) of (B) is fairly close to (D).
Gas (G) is produced by reacting K2MnF6 with:
View SolutionA crystalline solid (A) reacts with hydrogen gas to form a monoatomic gas (B) and a highly associated liquid ( C).(A) is soluble in water and undergoes hydroloysis slowly to form (B),(C ) and a diatomic gas (D).When pure and dry (D) is subjected to a silent electric discharge an another pale blue gas (E) is produced which like (A) also acts a strong oxidising agent.(E) dissolves in potassium hydroxide forming an orange coloured solid which is paramagnetic in nature. (A) reacts with IF_5, SbF_5, PF_5 etc to form the addition compounds. (C ) forms an addition compound (F) with KF with when electrolysed in molten state forms a most reactive gas (G).(B) and (G) (taken in 2:1) ratio when heated in nickel tube at 400^@ C and 6 atm pressure forms (A).The first ionization energy (IE_1) of (B) is fairly close to (D). Which of the following statements is correct for (A) ?
View SolutionA crystalline solid (A) reacts with hydrogen gas to form a monoatomic gas (B) and a highly associated liquid ( C).(A) is soluble in water and undergoes hydroloysis slowly to form (B),(C ) and a diatomic gas (D).When pure and dry (D) is subjected to a silent electric discharge an another pale blue gas (E) is produced which like (A) also acts a strong oxidising agent.(E) dissolves in potassium hydroxide forming an orange coloured solid which is paramagnetic in nature. (A) reacts with IF5,SbF5,PF5 etc to form the addition compounds. (C ) forms an addition compound (F) with KF with when electrolysed in molten state forms a most reactive gas (G).(B) and (G) (taken in 2:1) ratio when heated in nickel tube at 400∘C and 6 atm pressure forms (A).The first ionization energy (IE1) of (B) is fairly close to (D).
Which of the correct choice for (D) and (E) ?
View SolutionA crystalline solid (A) reacts with hydrogen gas to form a monoatomic gas (B) and a highly associated liquid ( C).(A) is soluble in water and undergoes hydroloysis slowly to form (B),(C ) and a diatomic gas (D).When pure and dry (D) is subjected to a silent electric discharge an another pale blue gas (E) is produced which like (A) also acts a strong oxidising agent.(E) dissolves in potassium hydroxide forming an orange coloured solid which is paramagnetic in nature. (A) reacts with IF5,SbF5,PF5 etc to form the addition compounds. (C ) forms an addition compound (F) with KF with when electrolysed in molten state forms a most reactive gas (G).(B) and (G) (taken in 2:1) ratio when heated in nickel tube at 400∘C and 6 atm pressure forms (A).The first ionization energy (IE1) of (B) is fairly close to (D).
Which of the following statements is true ?
View SolutionWhite crystalline solid (A) reacts with H2 to form a highly associated liquid (B) and a monoatomic , colourless gas (C ). The liquid (B) is used for etching glass. Compound (A) undergoes hydrolysis slowly to form (C ), (B) and a diatomic gas (D ) whose IE is almost similar to that of (C ). (B) forms an addition compound with KF to form (E) which is electrolysed in the molten state to form a most reactive gas (F) which combines with (C ) in 2:1 ratio of produce (A).
According to Molecular Orbital Theory, which of the following is correct about the molecule (D) ?
View SolutionWhite crystalline solid (A) reacts with H2 to form a highly associated liquid (B) and a monoatomic , colourless gas (C ). The liquid (B) is used for etching glass. Compound (A) undergoes hydrolysis slowly to form (C ), (B) and a diatomic gas (D ) whose IE is almost similar to that of (C ). (B) forms an addition compound with KF to form (E) which is electrolysed in the molten state to form a most reactive gas (F) which combines with (C ) in 2:1 ratio of produce (A).
Which of the following is correct for the white crystalline solid (A) ?
View SolutionWhite crystalline solid (A) reacts with H_2 to form a highly associated liquid (B) and a monoatomic , colourless gas (C ). The liquid (B) is used for etching glass. Compound (A) undergoes hydrolysis slowly to form (C ), (B) and a diatomic gas (D ) whose IE is almost similar to that of (C ). (B) forms an addition compound with KF to form (E) which is electrolysed in the molten state to form a most reactive gas (F) which combines with (C ) in 2:1 ratio of produce (A). The compound 'A' reacts with sulphur to form a compound in which hybridisation state of sulphur atom is :
View Solutionunderset"(Oxalic acid)"(H_2C_2O_4) oversetDeltato gas (A)+ gas (B) + liquid (C) Gas (A) burns with a blue flame and is oxidised to gas (B).Gas (B) turns lime water milky . Gas (A) + Cl_2 to (D) overset(NH_3 , Delta)to (E) A,B,C,D,E are respectively
View SolutionAn orange solid (A) on heating gave a green residue (B), colourless gas (C) and water vapour. The dry gas (C) on passing over heated magnesium gave a white solid (D).(D) on reaction with water have a gas (E) which formed dense white fumes with HCl. Indentify (A) to (E) and give the reactions.
View Solution

