Correct order of resonance energy of following compounds :
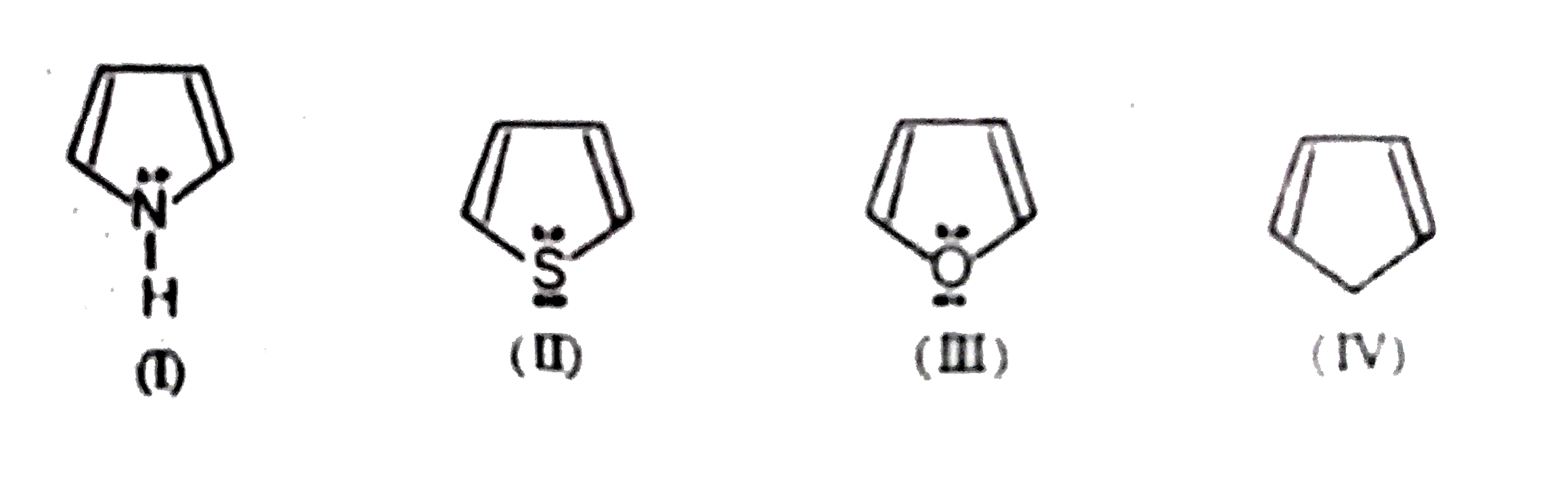
A
II>I>IV>III
B
II>IV>I>III
C
I>II>III>IV
D
II>I>III>IV
Video Solution
Text Solution
The correct Answer is:D
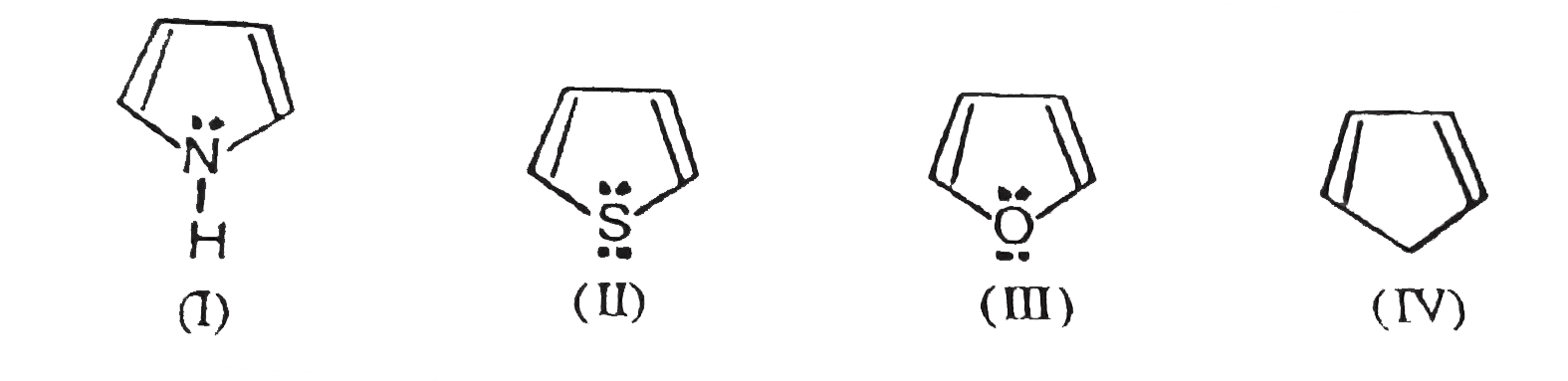
I,II,III are aromatic due to delocalisation so more resonance energy than IV.
Due to delocalisation- Ist get positive character on nitrogen
IInd get positive character on sulfur.
IIIrd get positive character on oxygen.
E.N: Oxygen gt Nitrogen gt Sulphur
So resonance energy IIgtIgtIIIgtIV.
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
Find out correct order of acidity of the following compounds :
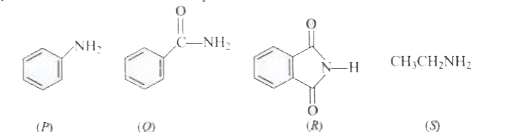 AR>Q>P>SBP>Q>R>SCR>Q>S>PDP>S>Q>R
AR>Q>P>SBP>Q>R>SCR>Q>S>PDP>S>Q>RCorrect order of reactivity of following compounds towards Grignard reagent?
 AI>II>IIIBII>I>IIICII>III>IDI>III>II
AI>II>IIIBII>I>IIICII>III>IDI>III>IIGive the correct order of increasing acidity of the following compounds.
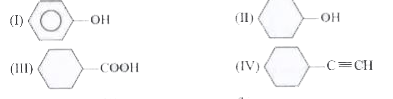 AII<I<IV<IIIBIV<II<I<IIICI<II<IV<IIIDIV<I<II<III
AII<I<IV<IIIBIV<II<I<IIICI<II<IV<IIIDIV<I<II<III
Correct order of bond energy is :
View SolutionThe correct order of dipole moment of the following compounds is

View SolutionWhich of the following is the correct order of stability of the
following compounds ?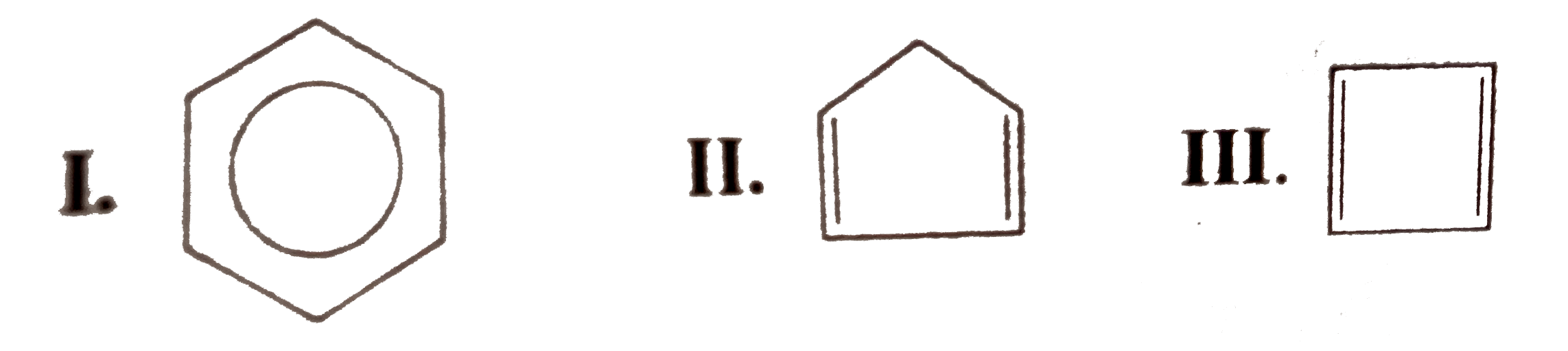
View Solutionthe correct order of basicities of the following compounds is
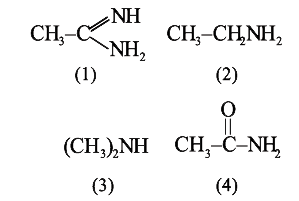
View SolutionThe correct order of strength of acidity of the following compounds is
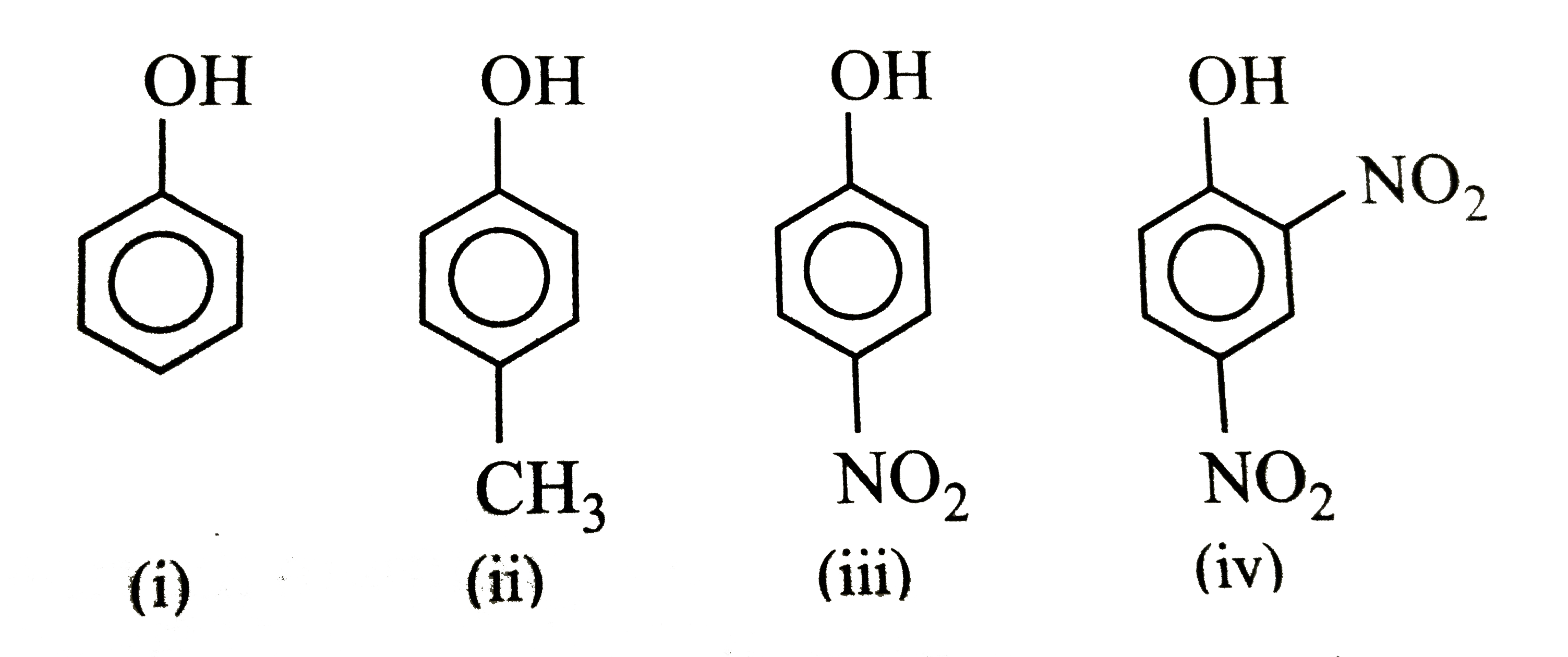
View Solution



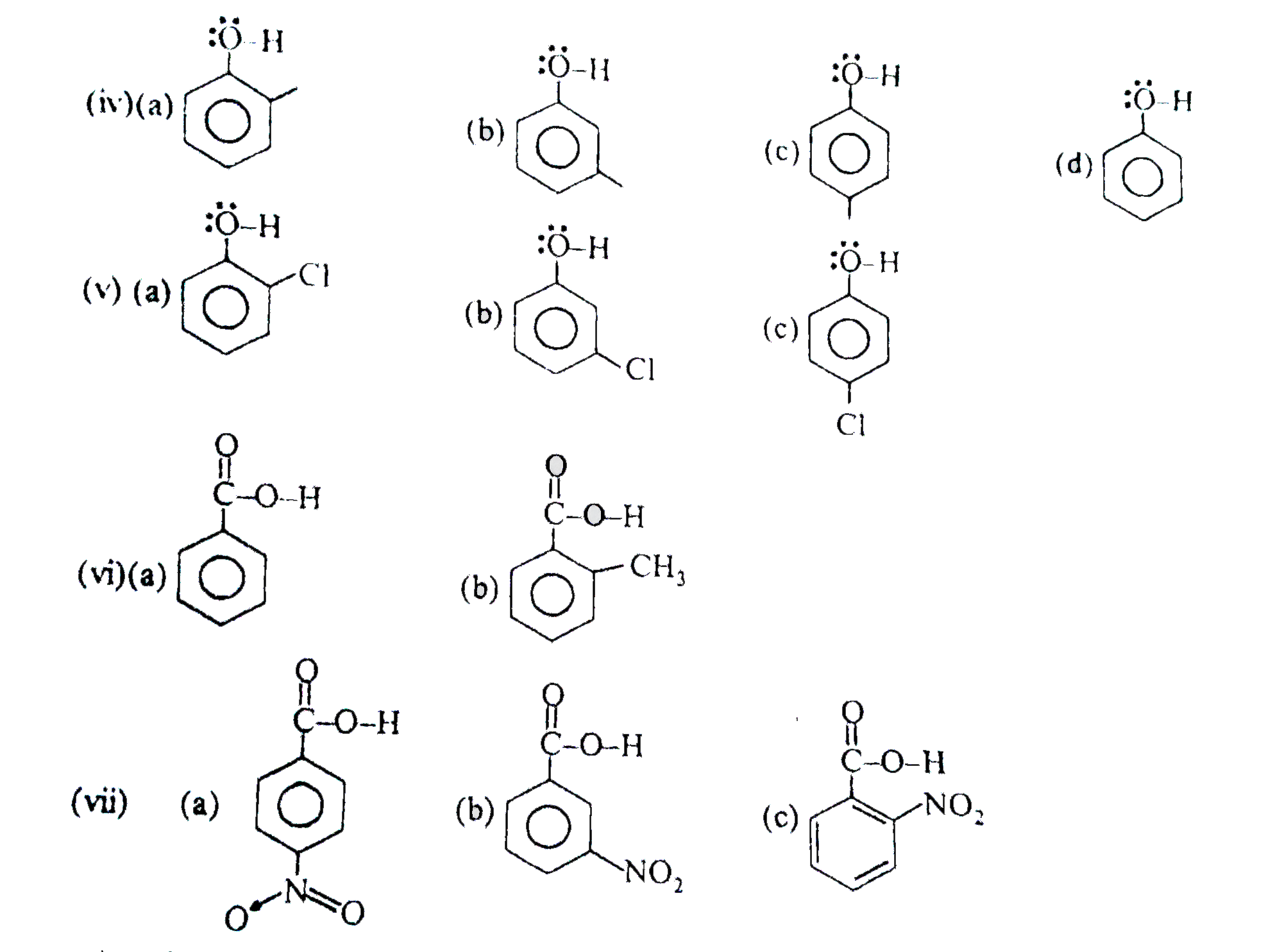 .
.