Column-IColumn-II(A)C2H5−O∣∣C−OH−C2H5OΘ−−−−−→(p)Hydrolysis(B)C2H5−O∣∣C−OH−⊕C2H5OH/H−−−−−−−→(q)Esterification(C)C2H5−O∣∣C−OC2H5H3O⊕−−−→(r)Saponification(D)C2H5−O∣∣C−OC2H5ΘOH−−→(s)Acid base solution
The correct Answer is:A→s ; B→q,s,t ; C→p,s,t ; D→p,r,t
To solve the question, we need to match the reactions from Column I with their corresponding types from Column II. Let's analyze each reaction step by step.
Step 1: Analyze Reaction A
- Reaction A: C2H5C(=O)OH + C2H5O^(-) → ?
- Here, we have an acid (C2H5C(=O)OH) reacting with an alkoxide ion (C2H5O^(-)).
- The product formed will be an anion (C2H5COO^(-)) and an alcohol (C2H5OH).
- This reaction can be classified as an acid-base reaction because it involves the transfer of a proton (H^+) from the acid to the alkoxide ion.
Conclusion for Reaction A:
- Match: A → S (Acid-base solution)
Step 2: Analyze Reaction B
- Reaction B: C2H5C(=O)OH + C2H5OH (in presence of H^+) → ?
- This is a classic esterification reaction where a carboxylic acid (C2H5C(=O)OH) reacts with an alcohol (C2H5OH) in the presence of an acid catalyst (H^+).
- The product formed will be an ester (C2H5C(=O)O-C2H5) and water.
Conclusion for Reaction B:
- Match: B → Q (Esterification)
Step 3: Analyze Reaction C
- Reaction C: C2H5C(=O)O-C2H5 + H3O^+ → ?
- This reaction involves the hydrolysis of an ester (C2H5C(=O)O-C2H5) in the presence of an acid (H3O^+).
- The products will be the corresponding carboxylic acid (C2H5C(=O)OH) and an alcohol (C2H5OH).
- This is a hydrolysis reaction.
Conclusion for Reaction C:
- Match: C → P (Hydrolysis)
Step 4: Analyze Reaction D
- Reaction D: C2H5C(=O)O-C2H5 + OH^(-) → ?
- In this case, the ester (C2H5C(=O)O-C2H5) is being hydrolyzed in the presence of a base (OH^(-)).
- This reaction is specifically known as saponification, where an ester reacts with a base to form a carboxylate ion and an alcohol.
Conclusion for Reaction D:
- Match: D → R (Saponification)
Final Matches:
- A → S (Acid-base solution)
- B → Q (Esterification)
- C → P (Hydrolysis)
- D → R (Saponification)
Summary of Matches:
- A - S
- B - Q
- C - P
- D - R
- Reaction A: C2H5C(=O)OH + C2H5O^(-) → ?
- Here, we have an acid (C2H5C(=O)OH) reacting with an alkoxide ion (C2H5O^(-)).
- The product formed will be an anion (C2H5COO^(-)) and an alcohol (C2H5OH).
- This reaction can be classified as an acid-base reaction because it involves the transfer of a proton (H^+) from the acid to the alkoxide ion.
- Match: A → S (Acid-base solution)
- Reaction B: C2H5C(=O)OH + C2H5OH (in presence of H^+) → ?
- This is a classic esterification reaction where a carboxylic acid (C2H5C(=O)OH) reacts with an alcohol (C2H5OH) in the presence of an acid catalyst (H^+).
- The product formed will be an ester (C2H5C(=O)O-C2H5) and water.
- Match: B → Q (Esterification)
- Reaction C: C2H5C(=O)O-C2H5 + H3O^+ → ?
- This reaction involves the hydrolysis of an ester (C2H5C(=O)O-C2H5) in the presence of an acid (H3O^+).
- The products will be the corresponding carboxylic acid (C2H5C(=O)OH) and an alcohol (C2H5OH).
- This is a hydrolysis reaction.
- Match: C → P (Hydrolysis)
- Reaction D: C2H5C(=O)O-C2H5 + OH^(-) → ?
- In this case, the ester (C2H5C(=O)O-C2H5) is being hydrolyzed in the presence of a base (OH^(-)).
- This reaction is specifically known as saponification, where an ester reacts with a base to form a carboxylate ion and an alcohol.
- Match: D → R (Saponification)
- A → S (Acid-base solution)
- B → Q (Esterification)
- C → P (Hydrolysis)
- D → R (Saponification)
- A - S
- B - Q
- C - P
- D - R
Topper's Solved these Questions
Similar Questions
A red solide is insoluble in water. However it becomes soluble if some KI is added to water. On heating the red solid in a test tube, there is liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The red solid is :
View SolutionSodium (Na =23) crystallizen in bcc arrangement with the interfacial separation between the atoms at the edge 53.6pm. The density of sodium crystal is:
View SolutionH−O∣∣C−OH and H3C−O∣∣C−OH can be distinguished by :
View SolutionConsidering the following reactions (I,II,III) and give your answer for following question.
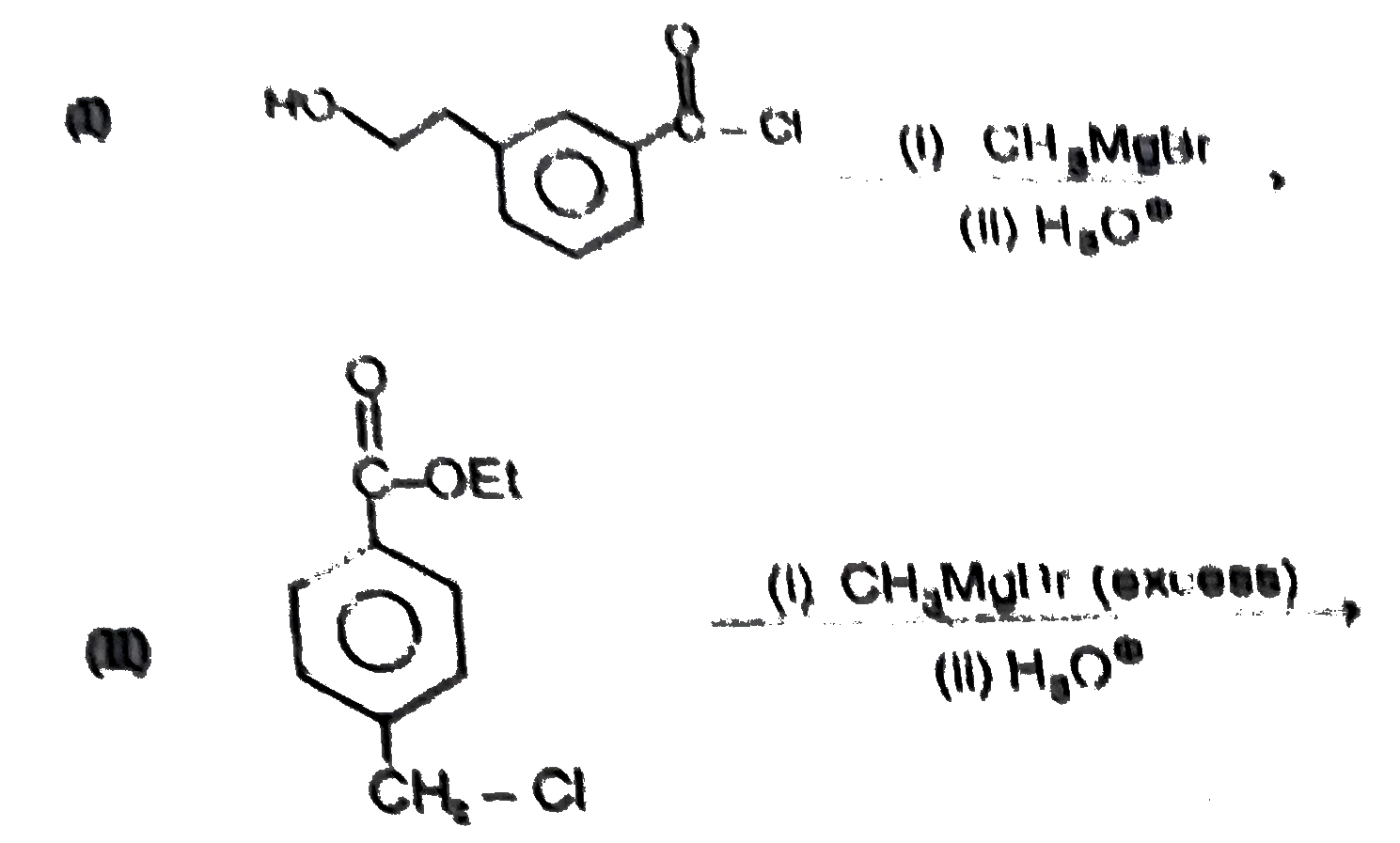
(i)CH3MgBr(excess)−−−−−−−−−−−−−→(ii)H3O⊕
(III)(a)EtO−O∣∣C−OET(i)PhMgBr(excess)−−−−−−−−−−−−→(ii)H+
(b)EtO−O∣∣C−OET(i)CH3MgBr(excess)−−−−−−−−−−−−−→(ii)H+
(c )H−O∣∣C−OET(i)PhMgBr(excess)−−−−−−−−−−−−→(ii)H+
(d)H−O∣∣C−OET(i)CH3MgBr(excess)−−−−−−−−−−−−−→(ii)H+
(e)ClCH2COOEt(i)CH3MgBr(excess)−−−−−−−−−−−−−→(ii)H+
The product in the reaction (II) will be :
View SolutionConsidering the following reactions (I,II,III) and give your answer for following question.
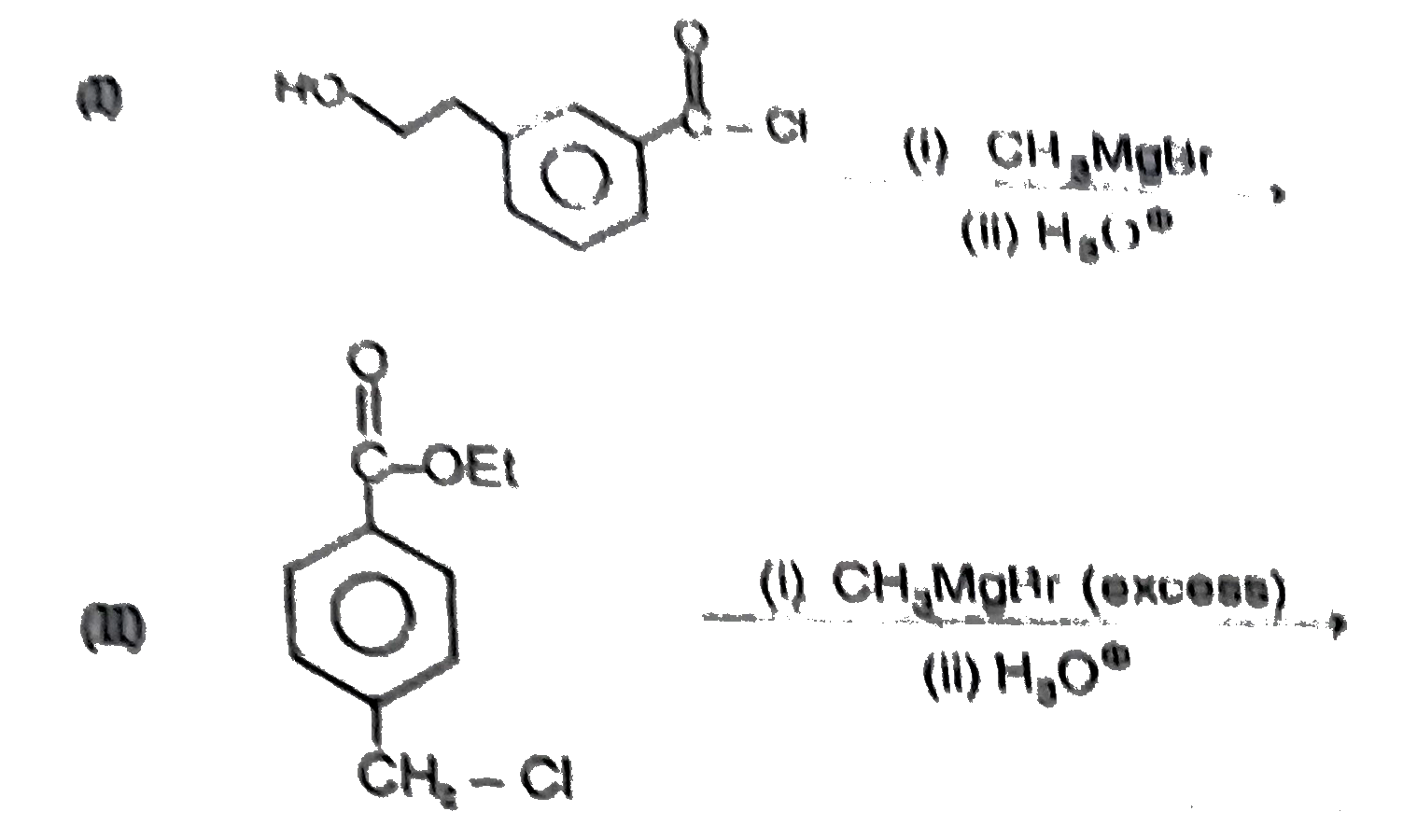
(i)CH3MgBr(excess)−−−−−−−−−−−−−→(ii)H3O⊕
(III)(a)EtO−O∣∣C−OET(i)PhMgBr(excess)−−−−−−−−−−−−→(ii)H+
(b)EtO−O∣∣C−OET(i)CH3MgBr(excess)−−−−−−−−−−−−−→(ii)H+
(c )H−O∣∣C−OET(i)PhMgBr(excess)−−−−−−−−−−−−→(ii)H+
(d)H−O∣∣C−OET(i)CH3MgBr(excess)−−−−−−−−−−−−−→(ii)H+
(e)ClCH2COOEt(i)CH3MgBr(excess)−−−−−−−−−−−−−→(ii)H+
In which set of the reactions in (III) the product will be 2∘ alcohol ?
View SolutionLactic acid CH_3-oversetoverset(OH)(|)C-CH-oversetoverset(O)(||)C-OH can be prepared by acid hydrolysis of reaction product of
View SolutionColumn-IColumn-II(A)C2H5−O∣∣C−OH−C2H5OΘ−−−−−→(p)Hydrolysis(B)C2H5−O∣∣C−OH−⊕C2H5OH/H−−−−−−−→(q)Esterification(C)C2H5−O∣∣C−OC2H5H3O⊕−−−→(r)Saponification(D)C2H5−O∣∣C−OC2H5ΘOH−−→(s)Acid base solution(t)Nucleophillic Substitution
View SolutionH−O∣∣C−OC2H5andCH3−O∣∣C−CH2−OH
Which is/are true about above two structure -
View SolutionExplain which compound is the weaker base.
O−−O∣∣C−O∣∣C−OH or HO−O∣∣C−O∣∣C−OH
View SolutionCH_3-overset(O)overset(||)C-OC_2 H_5+CH_3-overset(O)overset(||)C-O-C_2H_5 overset(C_2 H_5 O^(Theta) Na^(o+) C_2 H_5 OH)to . How many different products (including stereo)would beform by above reaction.
View Solution(I) CH_3 OH (II) C_2H_5OH (III) CH_3CH_2CH_2OH (IV) CH_3 -CH (OH)CH_3 (V) (CH_3)_3 C- OH (VI) CH_3 CH(OH)C_2H_5 (VII) CH_3COCH_3 (VIII) C C l_3 COCH_3 (IX) CH_3 CHO (X) CH_3 -overset( O) overset(||) C - OH (XI) CH_3 -overset( O) overset(||) C- O -C_2H_5 (XII) C_2 H_5-CHO Which of the above compounds cannot undergo iodoform reaction ?
View SolutionLactic acid CH3−OH∣CH−O∣∣C−OH can be prepared by acid hydrolysis of reaction product of
View SolutionH-oversetoverset(O)(||)C-OC_2H_5 "and" CH_3-oversetoverset(O)(||)C-CH_2-OH Which is/are true about above two structure -
View SolutionThe correct order of reactivity of PhMgBr with: (I) Ph-overset(O)overset(||)(C)-Ph (II) CH_(3)-oversetoverset(O)(||)(C)-H (III) CH_(3)-oversetoverset(O)(||)(C)-CH_(3) is:
View SolutionThe correct order of reactivity of PhMgBr with: (I) Ph-overset(O)overset(||)(C)-Ph (II) CH_(3)-oversetoverset(O)(||)(C)-H (III) CH_(3)-oversetoverset(O)(||)(C)-CH_(3) is:
View Solution

