How many moles of acetic anhydride (Ac2O) is needed to react completely with tartaric acid, ribose and glucose respectively,
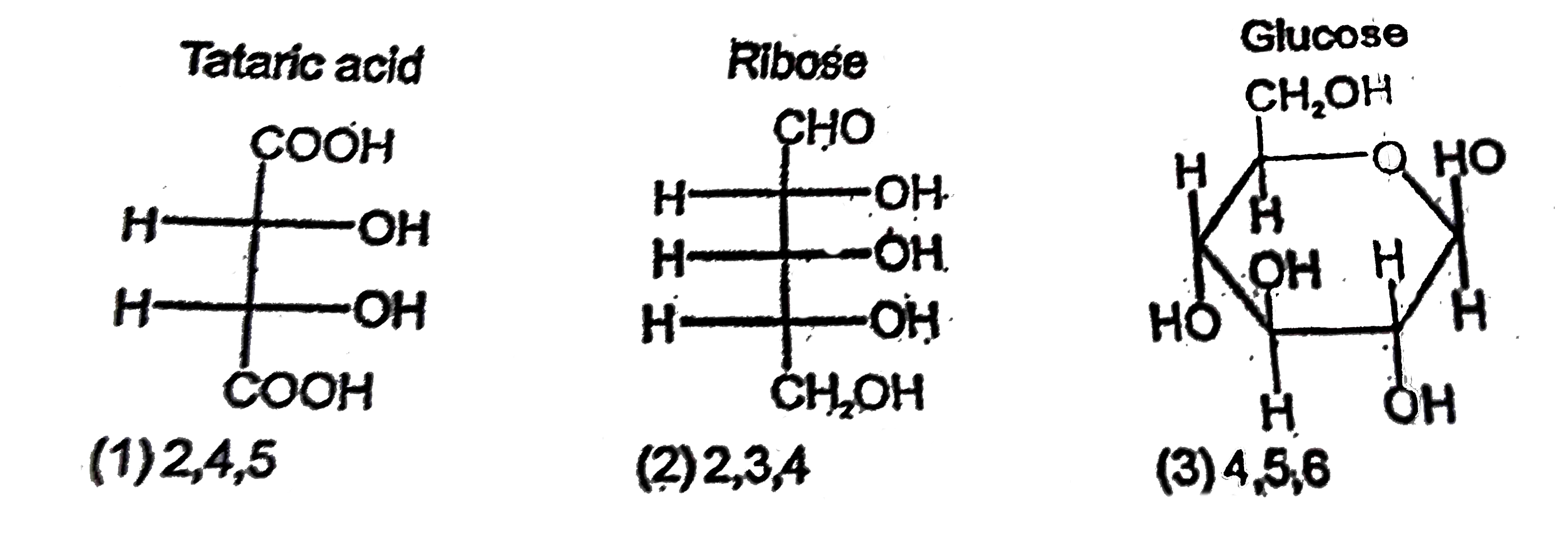
2,4,5
2,3,4
4,5,6
4,5,5
The correct Answer is:A
Depends upon number of -OH group for the formation of ester by acylation.
Topper's Solved these Questions
Similar Questions
A red solide is insoluble in water. However it becomes soluble if some KI is added to water. On heating the red solid in a test tube, there is liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The red solid is :
View SolutionSodium (Na =23) crystallizen in bcc arrangement with the interfacial separation between the atoms at the edge 53.6pm. The density of sodium crystal is:
View SolutionHow many moles of acetic anhydride (Ac_(2)O) is needed to react completely with tataric acid, ribose and glucose respectively,
View SolutionGlucose reacts with acetic anhydride to form
View SolutionHow many moles of acetic anhydride (Ac_2O) is required to react completely with 1 mole of sucrose ?
View SolutionHow many moles of acetic anhydride will be required to form glucose pentaacetate from 1 mole of glucose?
View SolutionHow many moles of acetic anhydride will be required to form glucose pentaacetate from 2 M of glucose ?
View SolutionHow many moles of acetic anhydride will be required to form glucose pertaacetate from 2M of glucose?
View SolutionHow many mole of acetic anhydride will consumed when it reacts with compound (A)
View SolutionGlucose reacts with acetic anhydride to form
View Solutionनिर्जल एसीटिक एनहाइड्राइड से क्रियाकर ग्लूकोज निर्मित करता है
View SolutionWith how many molecules of acetic anhydride does not molecule of glucose react?
View SolutionGlucose reacts with acetic anhydride to form
View SolutionGlucose reacts with acetic anhydride to from :
View SolutionGlucose reacts with acetic anhydride to from :
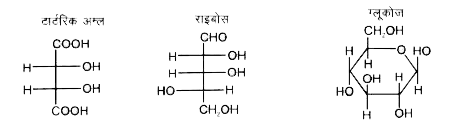
View Solutionटार्टरिक अम्ल, राइबोज और ग्लूकोज के साथ अभिक्रिया करने के लिए ऐसीटिक एनहाइड्राइड के क्रमश: कितने मोल की आवश्यकता होती है।
View Solution

