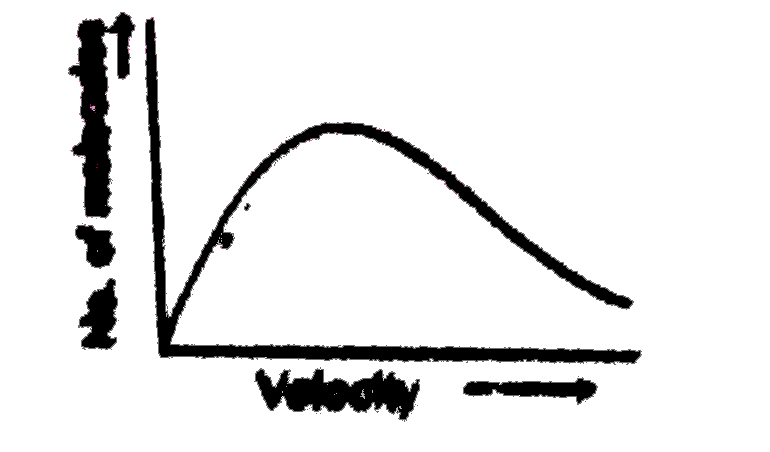
The velocity corresponding to a gas "X" (MW=100) corresponding to the maxima of the curve given below at a given temperature is 200 m/s.Then kinetic energy of 300 gram of has "X" will be
9000 kJ
9 kJ
90 kJ
810 kJ
The correct Answer is:B
The velocity corresponding to the maxima is the most probable velocity which is given by the expression
Cnp=√2RTM
RT=C2mpM2=(200)2×100×10−32=40000×50×10−3Jmol−1=40×50Jmol−1
No of moles =300100=3
E=32nRT=32×3×40×50=9×103J=9kJ
Topper's Solved these Questions
Similar Questions
A red solide is insoluble in water. However it becomes soluble if some KI is added to water. On heating the red solid in a test tube, there is liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The red solid is :
View SolutionSodium (Na =23) crystallizen in bcc arrangement with the interfacial separation between the atoms at the edge 53.6pm. The density of sodium crystal is:
View SolutionA gas consists of molecules of mass m and is at a temperature T . Making use of the Maxwell velocity distribution function, find the corresponding distribution of the molecules over the kinetic energies epsilon . Determine the most probable value of a gas the kinetic energy epsilon_p . Does epsilon_p correspond to the most probable velocity ?
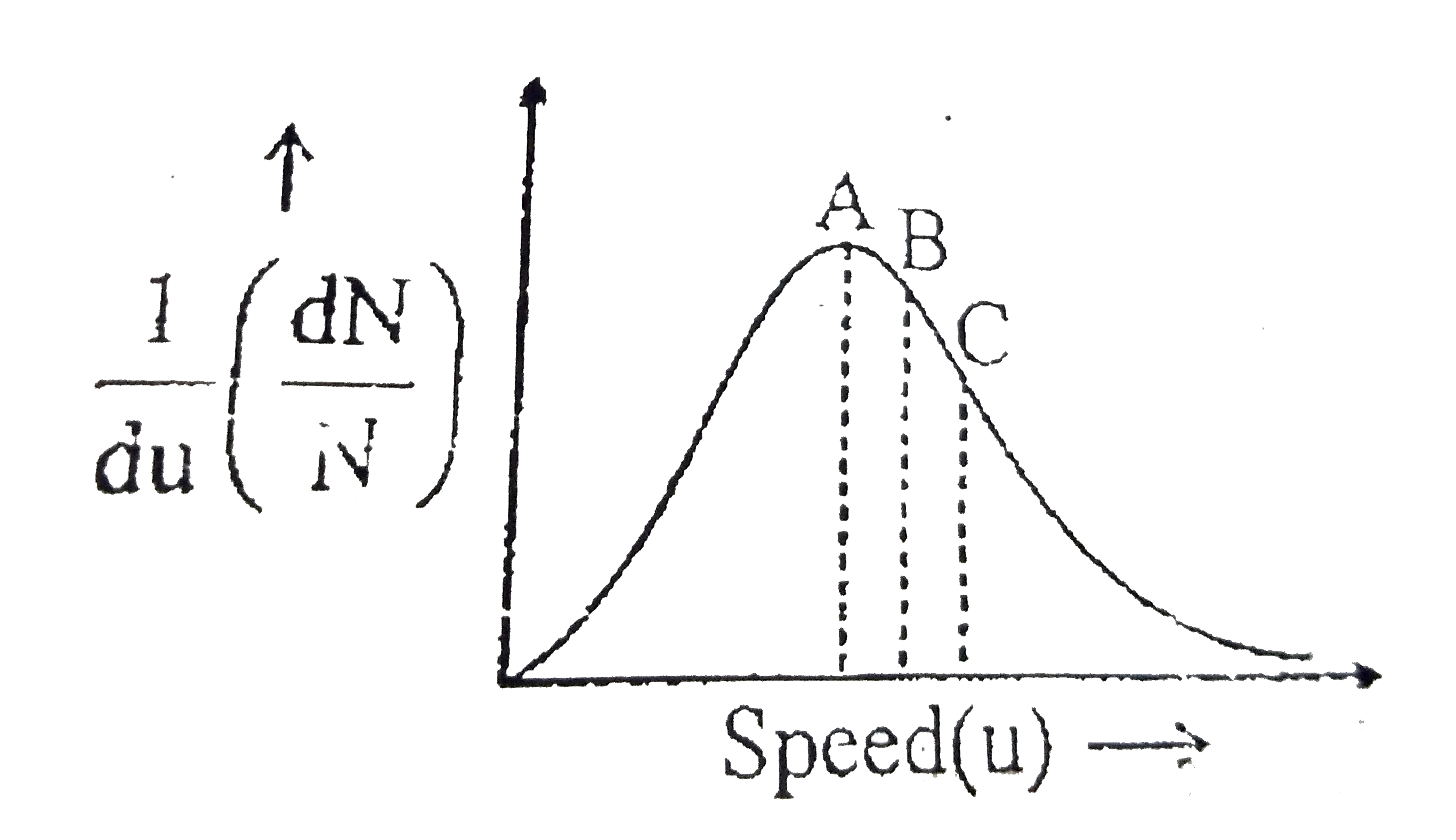
View SolutionThe slope of the curves near origin corresponding to energy distribution and speed distribution for an ideal gas is given by:
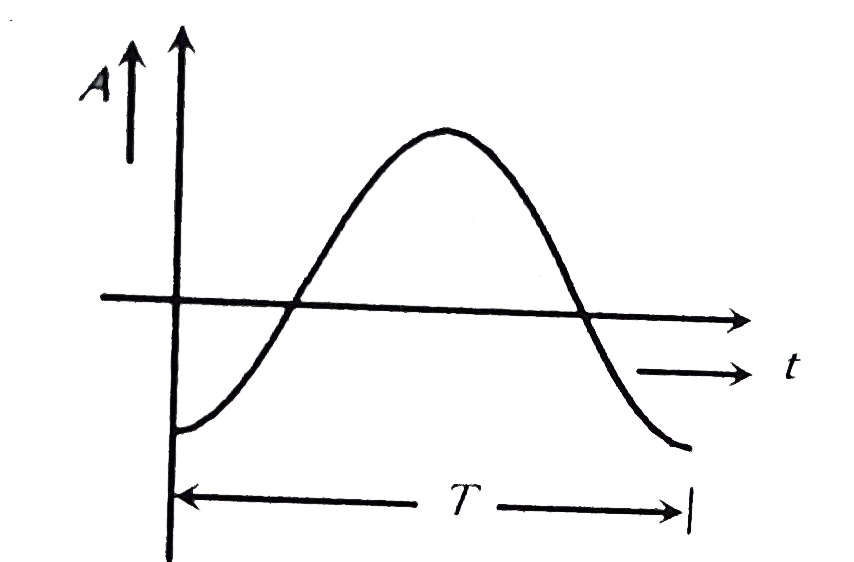
View SolutionAcceleration A and time period T of a body in S.H.M. is given by a curve shown below. Then corresponding graph, between kinetic energy (K.E.) and time t is correctly represented by
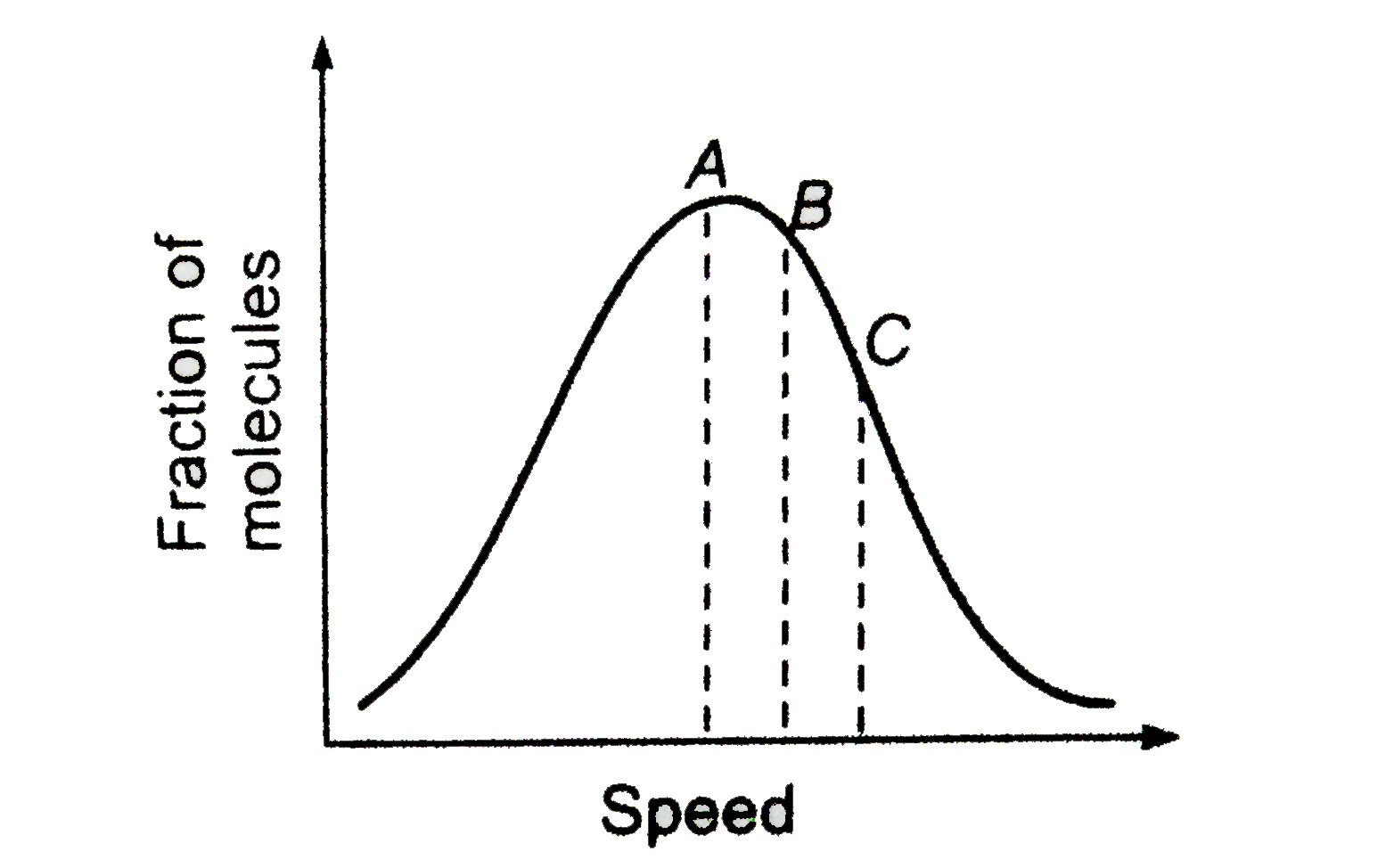
View SolutionAt a definit temperature (T), the distribution of speeds is given by the curve. In the curve points A, B and C indicates the speeds corresponding to :
View SolutionThe velocity corresponding to a gas "X" (MW=100) corresponding to the maxima of the curve given below at a given temperature is 200 m/s.Then kinetic energy of 300 gram of has "X" will be
View SolutionFor a sample of an ideal gas at given temperature (T), speed distribution curve is given as follows. Then the speeds corresponding to point A, B and C are respectively known as:
View SolutionThe potential energy diagram for a reaction X→Y is given. A and C in the graph corresponding to
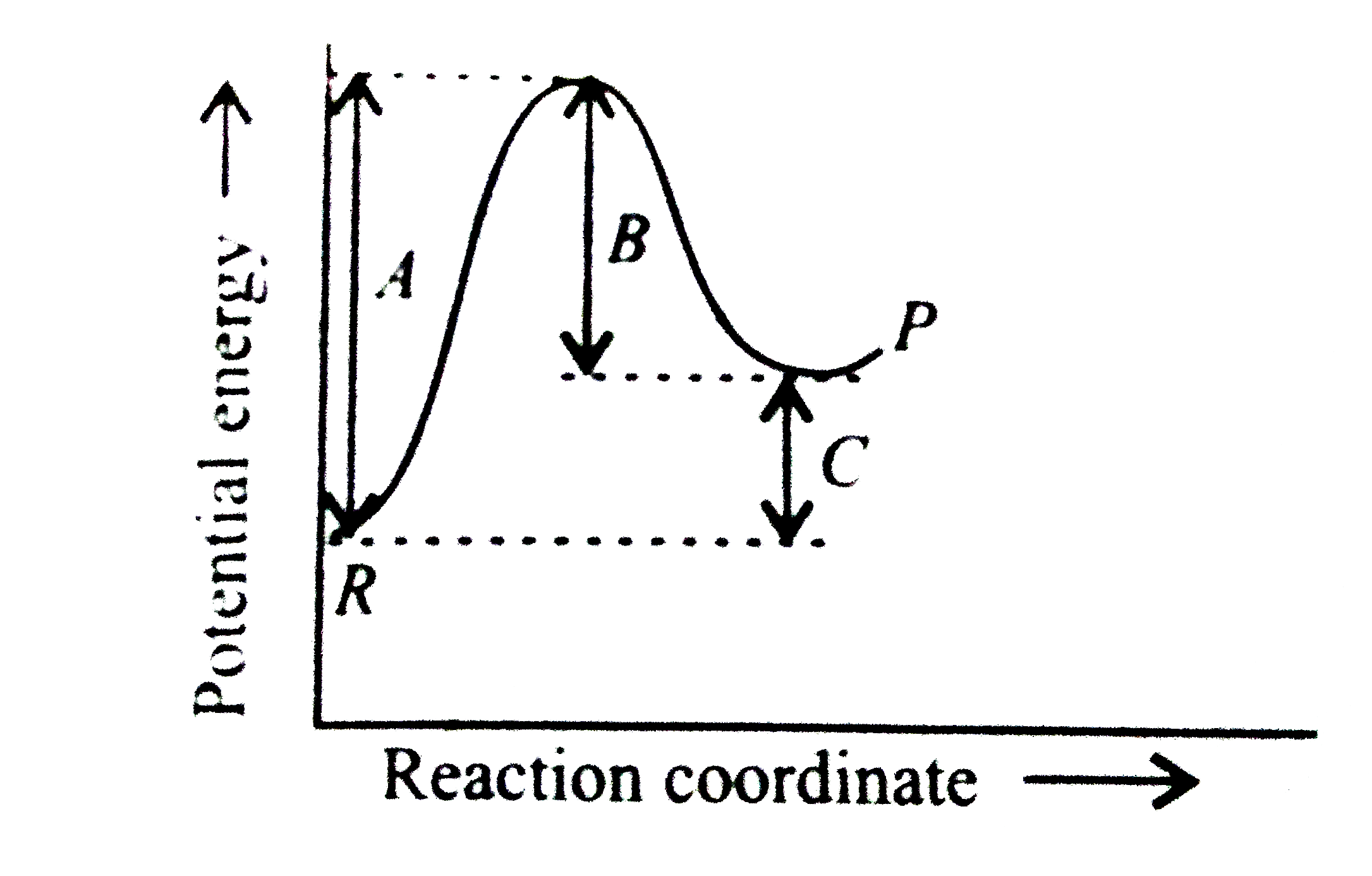
View SolutionAssertion (A): At a given temperature 1 mole of any gas has same kinetic energy.
Reason (R): Kinetic energy = (3/2)RT (for 1mole of gas).
View SolutionThe rms velocity of a gas at a given temperature is 300 m/s .What will be the rms velocity of a gas having twice the molecular weight and half the temperature in J
View SolutionThe rms velocity of a gas at a given temperature is 300 m/s. What will be the rms velocity of a gas having twice the molecular weight and half the temperature in K.
View SolutionThe potential energy diagram for a reaction X→Y is given. A and C in the graph corresponding to
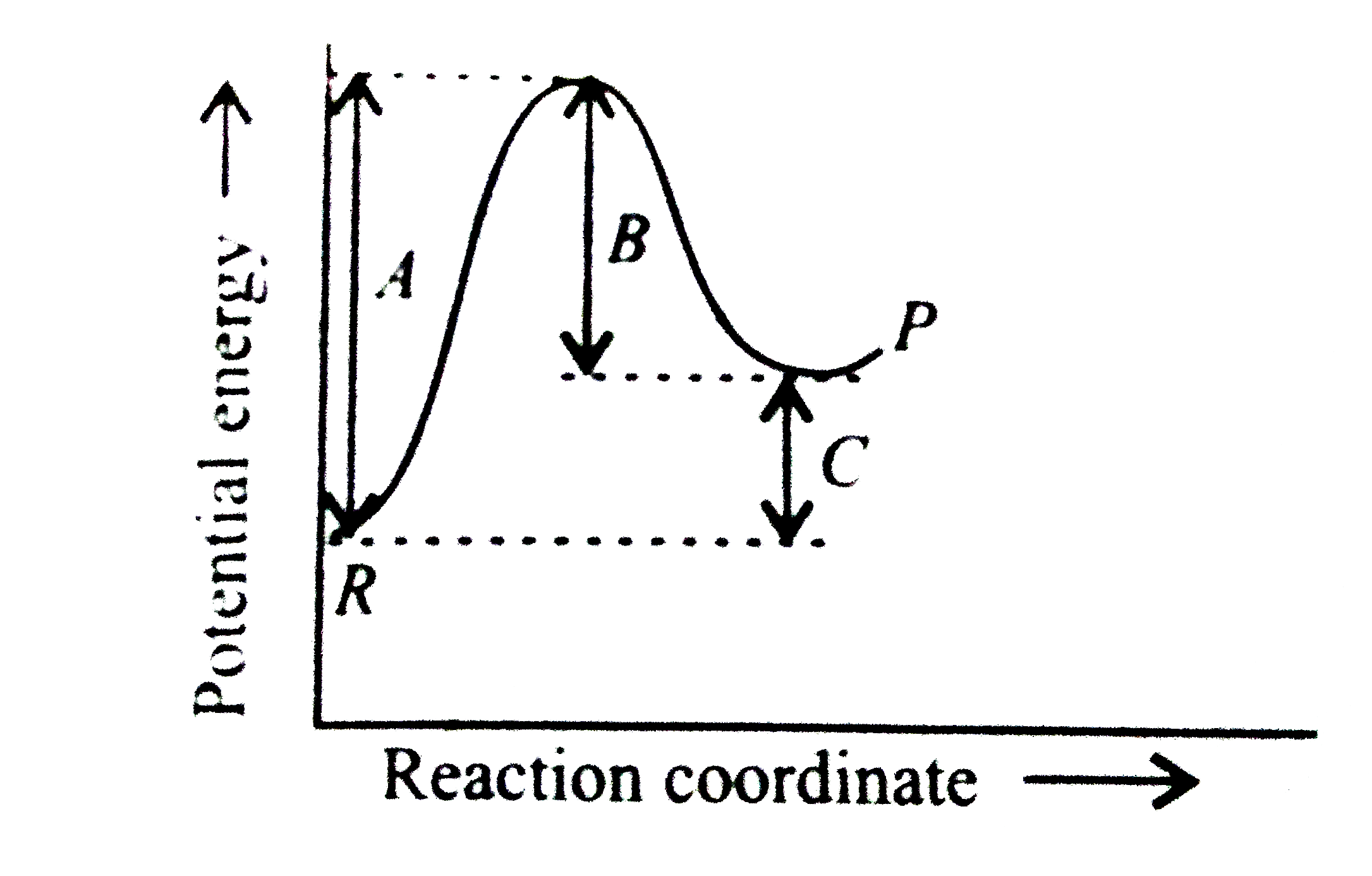
View SolutionFigure shows the pressure versus temperature curves for a given mass of a gas corresponding to two different volumes V_1 and V_2 then
View Solutionवृत्त की त्रिज्या 5 मी. हैं। उसका संगत व्यास निकालिए।
View Solutionकुछ वृत्तों की त्रिज्याएँ नीचे दी हुई हैं। उनके संगत व्यास निकालिए।
2.5 मी
View Solution

