Feo Cr2O3Na2CO3−−−−→O2AH2SO4−−−→BKCl−−→C
The hybridisation of compound C and colour of its crystal is
In all three compounds , the chromium is in +6 oxidation state
[Z] is a deep blue -violet coloured compound which decomposes rapidly in aqueous solution into Cr3+ and dioxygen
Saturated solution of [Y] gives bright organge compound , chromic anhydride, with concentrated H2SO4
All of these
The correct Answer is:D
4FeCr2O48Na2CO3+7O2Fusion−−−→+VINa2CrO4H+−−→H2O+VINa2Cr2O7H+/H2O2−−−−−−→+VICrO(O2)2("deep blue violet")overset(H_2O)toO_2+H_2O+Cr^(3+)<br>Na_2Cr_2O_7+H_2SO_4to2CrO_3("chromic anhydride")+Na_2SO_4+H_2O
Topper's Solved these Questions
Similar Questions
A red solide is insoluble in water. However it becomes soluble if some KI is added to water. On heating the red solid in a test tube, there is liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The red solid is :
View SolutionSodium (Na =23) crystallizen in bcc arrangement with the interfacial separation between the atoms at the edge 53.6pm. The density of sodium crystal is:
View SolutionPotassium dichromate (K2Cr2O7) is an orange coloured compound, very frequently used in laboratory as an oxidising agent as well as in a redox titration. It is generally prepared from chromit (FeCr2O4) ore according to the following reactions:
(a) Fusion of chromite ore with sodium carbonate in excess of air.
FeCr2O4+Na2CO3+O2→Na2CrO4+Fe2O3+CO2
(b) Acidifying filetered sodium chromate solution with sulphuric acid.
Na2CrO4+H2SO4→Na2Cr2O7+Na2SO4+H2O
(c) Treating sodium dichromate with potassium chloride.
Na2Cr2O7+KCl→K2Cr2O7+NaCl
Answer the following questions using above information.
If the number of moles of reactants available for reactions are: {FeCr2O4=0.25 moles, O2=0.35 moles, Na2CO3=0.60 moles, H2SO4=0.2 mol,es, KCl=0.1 moles}, then the maximum number of moles of K2Cr2O7, that can be produced is :
View SolutionPotassium dichromate (K2Cr2O7) is an orange coloured compound , very frequently used in laboratory as an oxidising agent as well as in a redox titration. It is generally prepared from chromite (FeCr2O4) ore according to the following reactions:
(1) Fusion of chromite ore with sodium carbonate in excess of air.
FeCr2O4+Na2CO3+O2→Na2CrO4+Fe2O3+CO2
(2) Acidifing filtered sodium chromate solution with sulphuric acid.
Na2CrO4+H2SO4→Na2Cr2O7+Na2SO4+H2O
(3) Treating sodium dichormate with potassium choride .
Na2Cr2O7+KCl→K2Cr2O7+NaCl
If the number of moles of reactant available for reaction are : [FeCr2O4=0.25moles,O2=0.35moles,Na2CO3=0.60moles,H2SO4=0.2moles,KCl=0.4moles] , then the maximum number of moles of K2Cr2O7 , that can be produced is :
View SolutionH3BO3375K−−→Ared Heat−−−−→B2O3
H3BO3435K−−→Bred Heat−−−−→B2O3
The compounds A & B are
View SolutionFeCr_(2)O_(4)+Na_(2)CO_(3)+O_(2)overset("Fusion")to[X]underset(H_(2)O)overset(H^(+))[Y]underset(H_(2)O_(2))overset(H^(+))to[Z] Whichf of the following statement is true for the compounds [X], [Y] and [Z]?
View SolutionFeO*Cr_(2)O_(3) underset(O_(2)) overset(Na_(2)CO_(3))to A overset(H_(2)SO_(4))toB overset(KCl)toC The hybridisation of compound C and colour of its crystal is
View SolutionConsider the following sequence of reactions
[A](C3H9N)NaNO2/HCl−−−−−−−→[B](C3H8O)K2Cr2O7−−−−−→H2SO4(C3H6O2)[C]
The compound [A] is
View SolutionFeCr2O4+NaOH+air→(A)+Fe2O3
(A)+(B)→Na2Cr2O7
Na2Cr2O7+XΔ−−→Cr2O3
Cr2O3+YΔ−−→Cr
Compound (A) and (B) are :
View SolutionFeCr2O4+NaOH+air→(A)+Fe2O3
(A)+(B)→Na2Cr2O7
Na2Cr2O7+XΔ−−→Cr2O3
Cr2O3+YΔ−−→Cr
(X) and (Y) are :
View SolutionFeCr2O4+NaOH+air→(A)+Fe2O3
(A)+(B)→Na2Cr2O7
Na2Cr2O7+XΔ−−→Cr2O3
Cr2O3+YΔ−−→Cr
Compound (A) and (B) are :
View SolutionFeCr2O4+NaOH+air→(A)+Fe2O3
(A)+(B)→Na2Cr2O7
Na2Cr2O7+XΔ−−→Cr2O3
Cr2O3+YΔ−−→Cr
(X) and (Y) are :
View SolutionIn the reaction, CH_(3)COOHoverset(NH_(3))toAoverset("Heat")toBoverset(P_(2)O_(5))toC C is :
View SolutionFeCr_(2)O_(4)+NaOH+"air" to (A)+Fe_(2)O_(3)(A)+(B) to Na_(2)Cr_(2)O_(7) Na_(2)Cr_(2)O_(7)+X overset(Delta) to Cr_(3),O_(3) Cr_(3)+Y(Delta) to Cr Compounds (A) and (B) are :
View Solutionनिम्नलिखित अभिक्रिया में H2SO4 का तुल्यांकी भार है
Na2Cr2O7+3SO2+H2SO4→3Na2SO4+Cr2(SO4)3+H2O
View SolutionIn the following reaction sequences, compounds A and D are respectively
Na2S2O3dil.H2SO4−−−−−→Na2SO4+S⏐⏐ ⏐↓+Bgas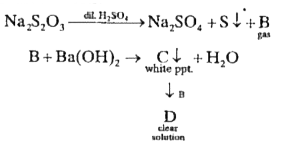
View Solution
