Consider the following species: P3−,S2−,CI−,K+,Ca2+ and Sc3+
(i) What is common in them?
(ii) Arrange them in the order of decreasing ionis radii.
The correct Answer is:(i) Each one of these ions contains 18 electrons and hence all are isoelectronic ions.
(ii) The ionic radii of isoelectronic ions decreases with the increases in the magnitude of the nuclear charge. For example, consider the isoelectronic ions :P3−,S2−,CI−,K'Ca2+ and Sc3+. All these ions have 18 electrons but their nuclear charges increase in the order: P3−(+15),S2−(+16),CI−(+17),K+(+19),Ca2+(+20)and Sc3+(+21). Therefore, their ionic radii decreases in the order: P3−>S2−>CI−>K+>Ca2+>Sc3+.
Step-by-Step Solution:
1. Identify the Species: The given species are P3−,S2−,Cl−,K+,Ca2+,Sc3+.
2. Determine the Atomic Numbers:
- Phosphorus (P): Atomic number 15
- Sulfur (S): Atomic number 16
- Chlorine (Cl): Atomic number 17
- Potassium (K): Atomic number 19
- Calcium (Ca): Atomic number 20
- Scandium (Sc): Atomic number 21
3. Calculate the Number of Electrons:
- For P3−: 15 + 3 = 18 electrons
- For S2−: 16 + 2 = 18 electrons
- For Cl−: 17 + 1 = 18 electrons
- For K+: 19 - 1 = 18 electrons
- For Ca2+: 20 - 2 = 18 electrons
- For Sc3+: 21 - 3 = 18 electrons
4. Conclusion: All these species have the same number of electrons (18 electrons). Therefore, they are isoelectronic species.
(ii) Arrange them in the order of decreasing ionic radii.
1. Understanding Ionic Radii: For isoelectronic species, the ionic radius is inversely proportional to the number of protons (atomic number). More protons result in a stronger nuclear charge, pulling the electrons closer and resulting in a smaller ionic radius.
2. List the Number of Protons:
- P3−: 15 protons
- S2−: 16 protons
- Cl−: 17 protons
- K+: 19 protons
- Ca2+: 20 protons
- Sc3+: 21 protons
3. Arrange by Number of Protons: Since we want to arrange them in decreasing order of ionic radii, we start with the species with the least number of protons:
- P3− (15 protons) > S2− (16 protons) > Cl− (17 protons) > K+ (19 protons) > Ca2+ (20 protons) > Sc3+ (21 protons)
4. Final Order: The order of decreasing ionic radii is:
P3−>S2−>Cl−>K+>Ca2+>Sc3+
2. Determine the Atomic Numbers:
- Phosphorus (P): Atomic number 15
- Sulfur (S): Atomic number 16
- Chlorine (Cl): Atomic number 17
- Potassium (K): Atomic number 19
- Calcium (Ca): Atomic number 20
- Scandium (Sc): Atomic number 21
3. Calculate the Number of Electrons:
- For P3−: 15 + 3 = 18 electrons
- For S2−: 16 + 2 = 18 electrons
- For Cl−: 17 + 1 = 18 electrons
- For K+: 19 - 1 = 18 electrons
- For Ca2+: 20 - 2 = 18 electrons
- For Sc3+: 21 - 3 = 18 electrons
4. Conclusion: All these species have the same number of electrons (18 electrons). Therefore, they are isoelectronic species.
2. List the Number of Protons:
- P3−: 15 protons
- S2−: 16 protons
- Cl−: 17 protons
- K+: 19 protons
- Ca2+: 20 protons
- Sc3+: 21 protons
3. Arrange by Number of Protons: Since we want to arrange them in decreasing order of ionic radii, we start with the species with the least number of protons:
- P3− (15 protons) > S2− (16 protons) > Cl− (17 protons) > K+ (19 protons) > Ca2+ (20 protons) > Sc3+ (21 protons)
4. Final Order: The order of decreasing ionic radii is:
P3−>S2−>Cl−>K+>Ca2+>Sc3+
Topper's Solved these Questions
- PERIODIC TABLE & PERIODICITYBOOK - RESONANCE ENGLISHCHAPTER - PERIODIC TABLE & PERIODICITYEXERCISE - Exercise-1
1 Video
- PERIODIC TABLE & PERIODICITYBOOK - RESONANCE ENGLISHCHAPTER - PERIODIC TABLE & PERIODICITYEXERCISE - Exercise
144 Videos
- PERIODIC TABLE & PERIODICITYBOOK - RESONANCE ENGLISHCHAPTER - PERIODIC TABLE & PERIODICITYEXERCISE - MISCELLANEOUS SOLVED PROBLEMS
7 Videos
- P-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)BOOK - RESONANCE ENGLISHCHAPTER - P-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)EXERCISE - APSP PART-3
22 Videos
- QUALITATIVE ANALYSISBOOK - RESONANCE ENGLISHCHAPTER - QUALITATIVE ANALYSISEXERCISE - INORGANIC CHMISTRY(Qualitative analysis)
35 Videos
Similar Questions
Knowledge Check
Consider the following species:
N3−,O2−,Fө,Na⊕,Mg2+ and Al3+
a. What is common in them?
b. Arrange them in the order of increasing ionic radii.AAll have same ionic radii.BAll are isoelectronic species having 10 electrons.CAll of them belong to the third period.DThe nature of oxides of all the ions is basic.Consider the following species :
(A) өF (B) CH3−өO (C) өCH3 (D) өNH2
Arrange these species in their decreasing order of nucleophilicity.AC>D>B>ABB>A>C>DCA>B>C>DDC>A>B>D
Consider the ioselectronic series , K⊕,S2−,Clɵ,Ca2+, the radii of the ions decrease as
View Solutionwhich of the following species are isoelectronic ?
(i) Ne ,(ii) O2− , (iii) Mg2+ , (iv) F
(v) Al3+ , (vi) Clө , (vii) K , (viii) Na
Arrange them in decreasing order of their size.
View SolutionAmong IE1,IE2,IE3 of an element, which is the largest ? Arrange them in the increasing order
View SolutionConsider the following perhalate ions in acidic medium ClO−4(I),BrO−4(II),IO−4(III)
Arrange these in the decreasing order of oxidizing power
View SolutionThe following reaction occurs at 700K. Arrange them in the order of increasing tendency to proceed to completion.
I.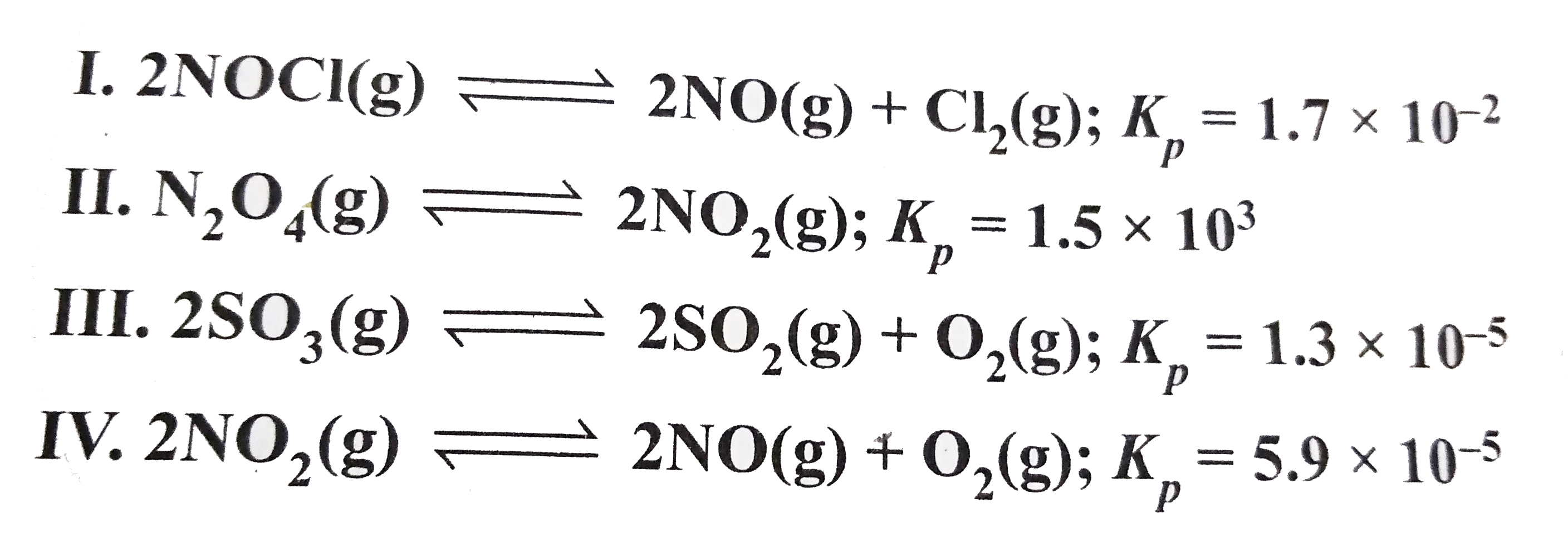
View SolutionArrange the following in the order of their increasing size : Cl−,S2−,Ca2+,Ar
View Solution

