The first ionisation enthalpies (in eV) of N & O are respectively given by:
14.6, 13.6
13.6, 14.6
13.6, 13.6
14.6, 14.6
The correct Answer is:A
Step by step video solution for The first ionisation enthalpies (in eV) of N & O are respectively given by: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- PERIODIC TABLE & PERIODICITYBook:RESONANCE ENGLISHChapter:PERIODIC TABLE & PERIODICITYExercise:Exercise-2
Explore 1 Video
- PERIODIC TABLE & PERIODICITYBook:RESONANCE ENGLISHChapter:PERIODIC TABLE & PERIODICITYExercise:Exercise-3
Explore 1 Video
- PERIODIC TABLE & PERIODICITYBook:RESONANCE ENGLISHChapter:PERIODIC TABLE & PERIODICITYExercise:Exercise-1
Explore 1 Video
- P-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)Book:RESONANCE ENGLISHChapter:P-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)Exercise:APSP PART-3
Explore 22 Videos
- QUALITATIVE ANALYSISBook:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSISExercise:INORGANIC CHMISTRY(Qualitative analysis)
Explore 35 Videos
Similar Questions
Ionisation Enthalpy
View SolutionThe first ionisation energies of magnesium and aluminium are respectively given by
View SolutionThe first ionisation potential is maximum for
View SolutionThe first ionisation potentials (eV) of Be and B respectively are
View SolutionThe first ionization potential (eV) of N and P respectively are
View SolutionThe first ionisation energy is maximum for
View SolutionThe electron gain enthalpy (in kJ/mole) of F, O, N and C respectively are :
View SolutionCorrect order of ionisation enthalpy is
View SolutionThe first ionisation enthalpies of four consecutive elements present in the second period of the periodic table are 8.3, 11.3, 14.5 and 13.6 eV respectively. Which one of the following is the first ionisation enthalpy of nitrogen ?
View SolutionFirst and second ionisation enthalpies(in KJ/mol) of few elements are given below:
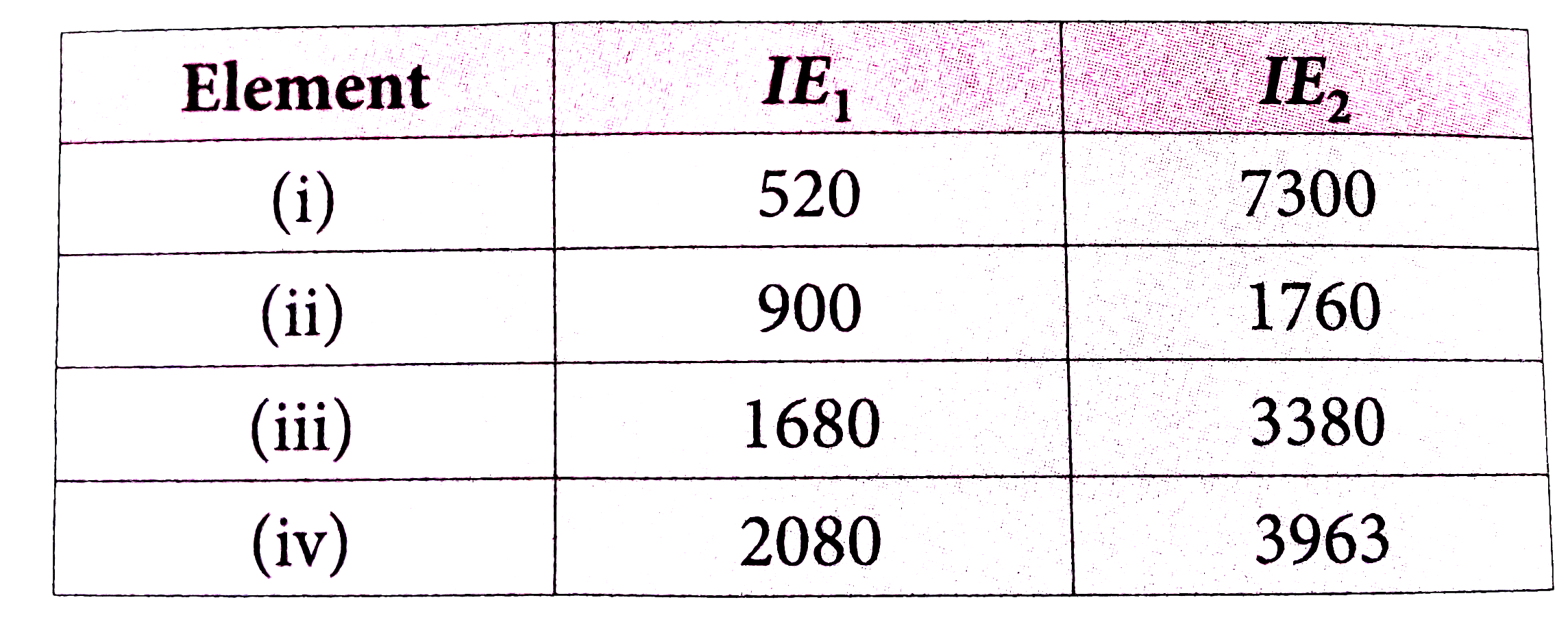
Which of the above elements will form halides with formula MX2?
View Solution
