The group that has the species correctly listed in the order of decreasing radius is:
cu2+,cu+,Cu
V,V2+,V3+
F−,Br−,I
B,Be,Li
The correct Answer is:B
To solve the question regarding the order of decreasing atomic radius for the given species, we need to understand the trends in atomic and ionic radii in the periodic table.
1. Understand the Trends in Atomic Radius:
- Atomic radius decreases across a period from left to right due to increasing nuclear charge, which pulls the electrons closer to the nucleus.
- Atomic radius increases down a group due to the addition of electron shells.
2. Consider the Effect of Ionization:
- Cations (positively charged ions) are smaller than their parent atoms because they lose one or more electrons, resulting in less electron-electron repulsion.
- Anions (negatively charged ions) are larger than their parent atoms because they gain electrons, increasing electron-electron repulsion.
3. Analyze the Given Options:
- Option 1: Cu²⁺, Cu⁺, Cu
- Cu²⁺ < Cu⁺ < Cu (increasing order, not decreasing).
- Option 2: V, V²⁺, V³⁺
- V > V²⁺ > V³⁺ (correct order of decreasing radius).
- Option 3: F⁻, Br⁻, I
- I > Br⁻ > F⁻ (Iodine has the largest radius, but the order is not correct as it should be I > Br⁻ > F⁻).
- Option 4: B, Be, Li
- Li > Be > B (this is incorrect as they are in the same period, and the order should be Be < B < Li).
4. Conclusion:
- The only option that correctly lists the species in the order of decreasing radius is Option 2: V, V²⁺, V³⁺.
Final Answer:
The group that has the species correctly listed in the order of decreasing radius is Option 2: V, V²⁺, V³⁺.
- Atomic radius decreases across a period from left to right due to increasing nuclear charge, which pulls the electrons closer to the nucleus.
- Atomic radius increases down a group due to the addition of electron shells.
- Cations (positively charged ions) are smaller than their parent atoms because they lose one or more electrons, resulting in less electron-electron repulsion.
- Anions (negatively charged ions) are larger than their parent atoms because they gain electrons, increasing electron-electron repulsion.
- Option 1: Cu²⁺, Cu⁺, Cu
- Cu²⁺ < Cu⁺ < Cu (increasing order, not decreasing).
- Option 2: V, V²⁺, V³⁺
- V > V²⁺ > V³⁺ (correct order of decreasing radius).
- Option 3: F⁻, Br⁻, I
- I > Br⁻ > F⁻ (Iodine has the largest radius, but the order is not correct as it should be I > Br⁻ > F⁻).
- Option 4: B, Be, Li
- Li > Be > B (this is incorrect as they are in the same period, and the order should be Be < B < Li).
- The only option that correctly lists the species in the order of decreasing radius is Option 2: V, V²⁺, V³⁺.
The group that has the species correctly listed in the order of decreasing radius is Option 2: V, V²⁺, V³⁺.
Topper's Solved these Questions
- PERIODIC TABLE & PERIODICITYBook:RESONANCE ENGLISHChapter:PERIODIC TABLE & PERIODICITYExercise:Exercise-3
Explore 1 Video
- P-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)Book:RESONANCE ENGLISHChapter:P-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)Exercise:APSP PART-3
Explore 22 Videos
- QUALITATIVE ANALYSISBook:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSISExercise:INORGANIC CHMISTRY(Qualitative analysis)
Explore 35 Videos
Similar Questions
Knowledge Check
The species listed in Red Data Book are
AThreatenedBEndangeredCRareDAll of these
The reducing power of divalent species decreases in the order
View SolutionThe group having isoelectronic species is
View SolutionThe group fungi has more species than all the vertebrate species combined.
View SolutionAssertion: In the case of polyfunctional compounds, the choice of principal functional group is made on the basis of order of preference.
Reason: The order of decreasing priority for some functional group is
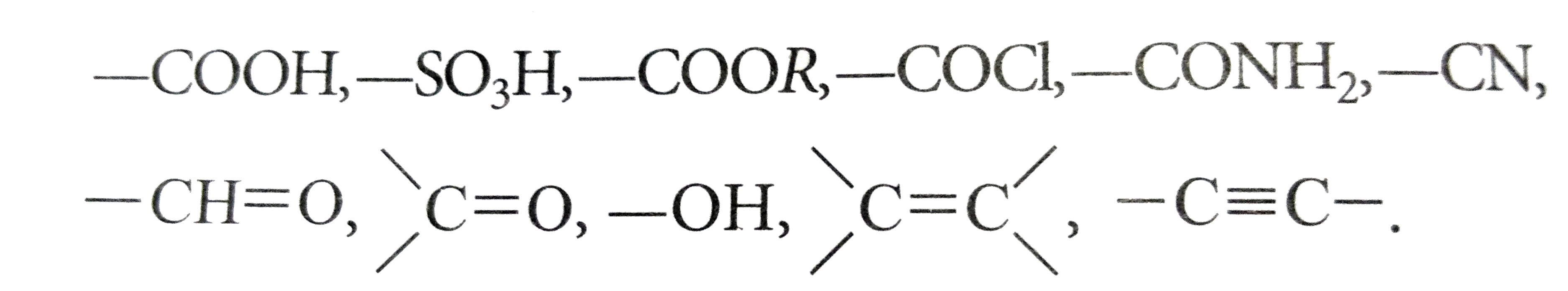
View SolutionWhen the following five anions are arranged in order of decreasing ionic radius , the correct sequence is :
View Solution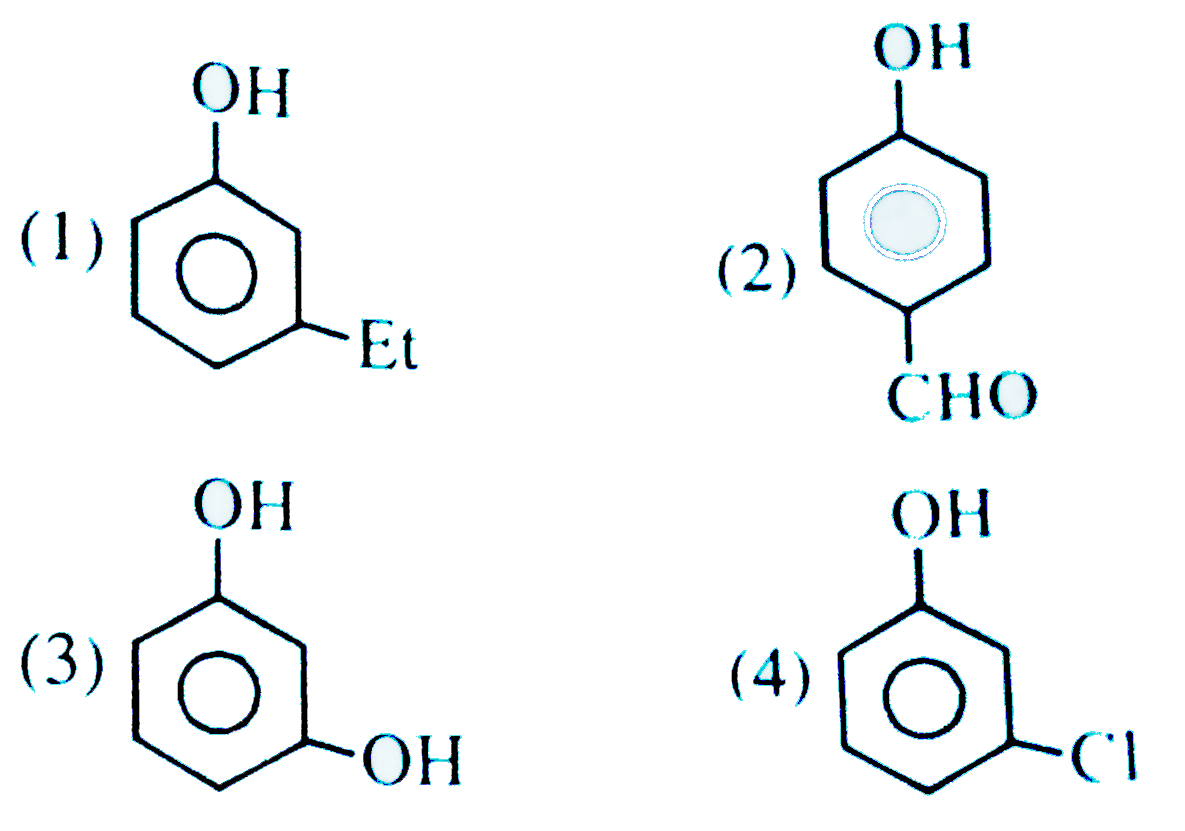
Decreasing order of pKa is :
View SolutionArrange the given species in the increasing order of Leaving group ability
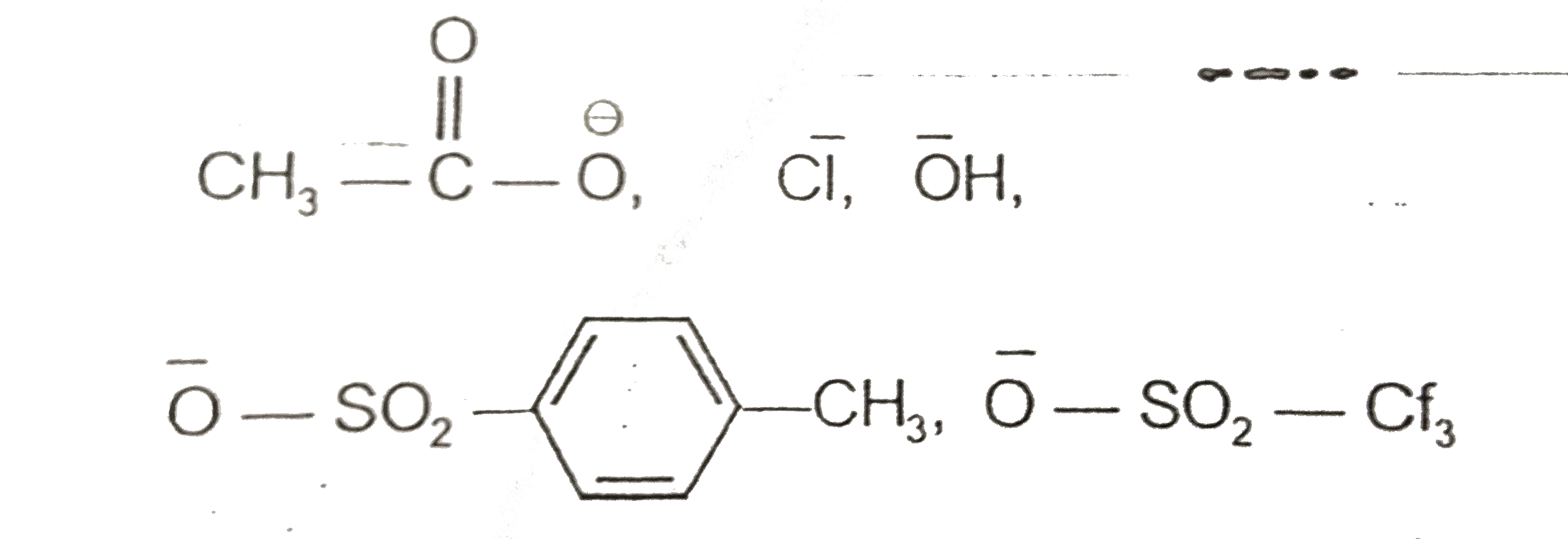
View Solution

