Which of the following IUPAC names are correct.
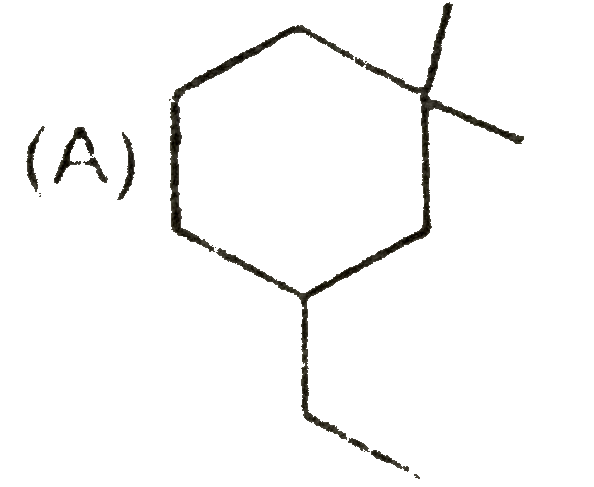
3-Ethyl-1,1-dimethylcycohexane
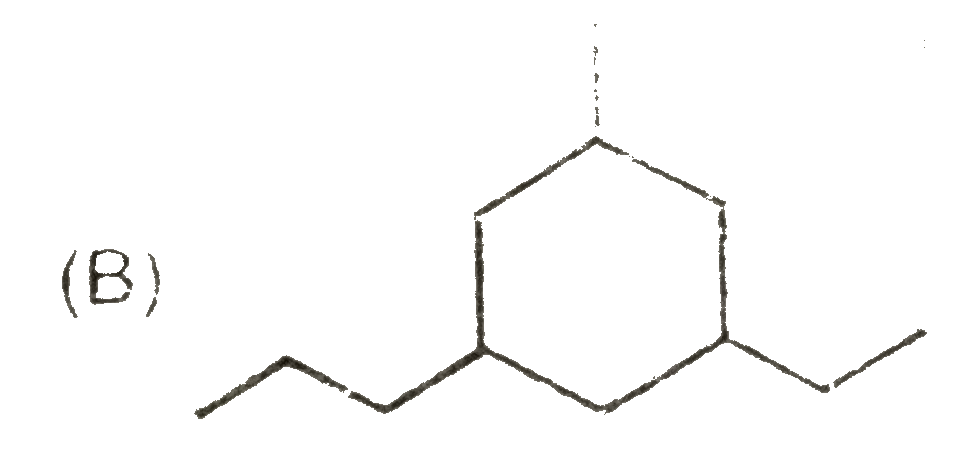
1-Ethyl-3-methyl-5-propylcyclohexane
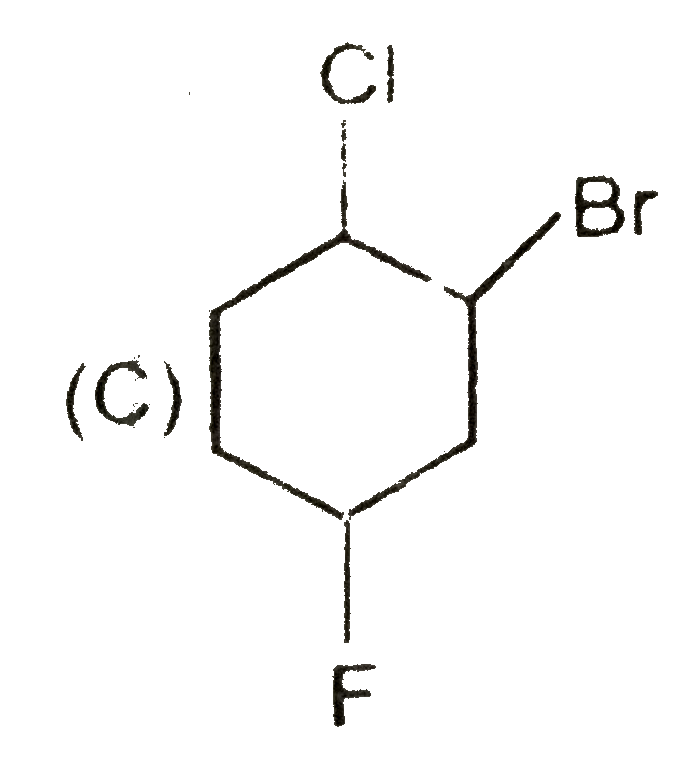
2-Bromo-1-chloro-4-fluorocyclohexane
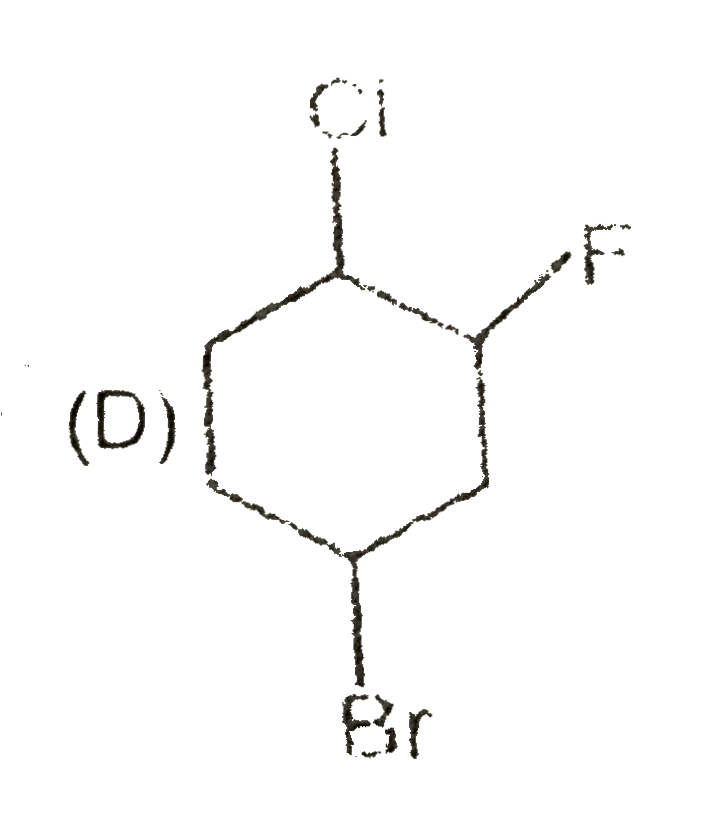
1-Bromo-4-chloro-3-fluorocyclohexane
The correct Answer is:A, B, C
To determine which of the provided IUPAC names are correct, we will analyze each option step by step based on the IUPAC nomenclature rules.
1. Identify the Structure:
- For each option, we need to visualize or draw the molecular structure based on the given name. This helps in understanding the arrangement of carbon atoms, functional groups, and substituents.
2. Numbering the Carbon Chain:
- Start numbering the carbon atoms in the longest continuous chain or ring. The numbering should prioritize the functional groups and the substituents to get the lowest possible numbers.
3. Check for Functional Groups:
- Identify if there are any functional groups present in the structure. Functional groups take precedence in naming.
4. Identify Unsaturation:
- Check for double or triple bonds (unsaturation). If present, they should be indicated in the name with the appropriate suffix (e.g., -ene for double bonds, -yne for triple bonds).
5. Identify Substituents:
- Identify all substituents (alkyl groups, halogens, etc.) attached to the main chain.
6. Assign Locants to Substituents:
- Assign numbers to the substituents based on their position on the carbon chain. If there are multiple substituents of the same type, use prefixes (di-, tri-, etc.) and list them in alphabetical order when writing the name.
7. Construct the IUPAC Name:
- Combine the substituents and the main chain name according to the rules. Ensure to place substituents in alphabetical order regardless of their position numbers.
8. Verify Each Option:
- For each IUPAC name provided, check if the constructed name matches the original name given in the options.
Analysis of Each Option:
- Option 1: 3-Ethyl-1,1-dimethylcyclohexane
- Correctly identifies the cyclic structure with substituents. The numbering gives the lowest locants to the substituents. This name is correct.
- Option 2: 1-Ethyl-3,3-dimethylcyclohexane
- The numbering is done correctly, and substituents are in the correct order. This name is correct.
- Option 3: 2-Bromo-1-chloro-1-fluorocyclohexane
- The numbering gives the lowest locants to the substituents, and the alphabetical order is maintained. This name is correct.
- Option 4: 4-Bromo-1-chloro-2-fluorocyclohexane
- The alphabetical order is not maintained as "Bromo" should come after "Chloro." This name is incorrect.
Conclusion:
The correct IUPAC names are from options 1, 2, and 3. Option 4 is incorrect.
- For each option, we need to visualize or draw the molecular structure based on the given name. This helps in understanding the arrangement of carbon atoms, functional groups, and substituents.
- Start numbering the carbon atoms in the longest continuous chain or ring. The numbering should prioritize the functional groups and the substituents to get the lowest possible numbers.
- Identify if there are any functional groups present in the structure. Functional groups take precedence in naming.
- Check for double or triple bonds (unsaturation). If present, they should be indicated in the name with the appropriate suffix (e.g., -ene for double bonds, -yne for triple bonds).
- Identify all substituents (alkyl groups, halogens, etc.) attached to the main chain.
- Assign numbers to the substituents based on their position on the carbon chain. If there are multiple substituents of the same type, use prefixes (di-, tri-, etc.) and list them in alphabetical order when writing the name.
- Combine the substituents and the main chain name according to the rules. Ensure to place substituents in alphabetical order regardless of their position numbers.
- For each IUPAC name provided, check if the constructed name matches the original name given in the options.
- Correctly identifies the cyclic structure with substituents. The numbering gives the lowest locants to the substituents. This name is correct.
- The numbering is done correctly, and substituents are in the correct order. This name is correct.
- The numbering gives the lowest locants to the substituents, and the alphabetical order is maintained. This name is correct.
- The alphabetical order is not maintained as "Bromo" should come after "Chloro." This name is incorrect.
The correct IUPAC names are from options 1, 2, and 3. Option 4 is incorrect.
Topper's Solved these Questions
- IUPAC NOMENCLATURE & STRUCTURAL ISOMERISM Book:RESONANCE ENGLISHChapter:IUPAC NOMENCLATURE & STRUCTURAL ISOMERISM Exercise:Exercise-2 Part-4
Explore 2 Videos
- IUPAC NOMENCLATURE & STRUCTURAL ISOMERISM Book:RESONANCE ENGLISHChapter:IUPAC NOMENCLATURE & STRUCTURAL ISOMERISM Exercise:Exercise-3 Part-1
Explore 9 Videos
- IUPAC NOMENCLATURE & STRUCTURAL ISOMERISM Book:RESONANCE ENGLISHChapter:IUPAC NOMENCLATURE & STRUCTURAL ISOMERISM Exercise:Exercise-2 Part-2
Explore 11 Videos
- IONIC EQUILIBRIUMBook:RESONANCE ENGLISHChapter:IONIC EQUILIBRIUMExercise:partIII one or more than one options correct type
Explore 10 Videos
- METALLURGYBook:RESONANCE ENGLISHChapter:METALLURGYExercise:ORGANIC CHEMISTRY(Alkyl Halide, Alcohol,Phenol,Ether)
Explore 17 Videos
Similar Questions
There are three isomeric compounds P,Q,R with molecular formula C4H6O3.Compound P is a saturated hydroxy carboxylic acid.Compound Q is a symmetrical anhydride while R is an aldehydic ester.
Which of the following is P?
View SolutionWrite IUPAC name of the following
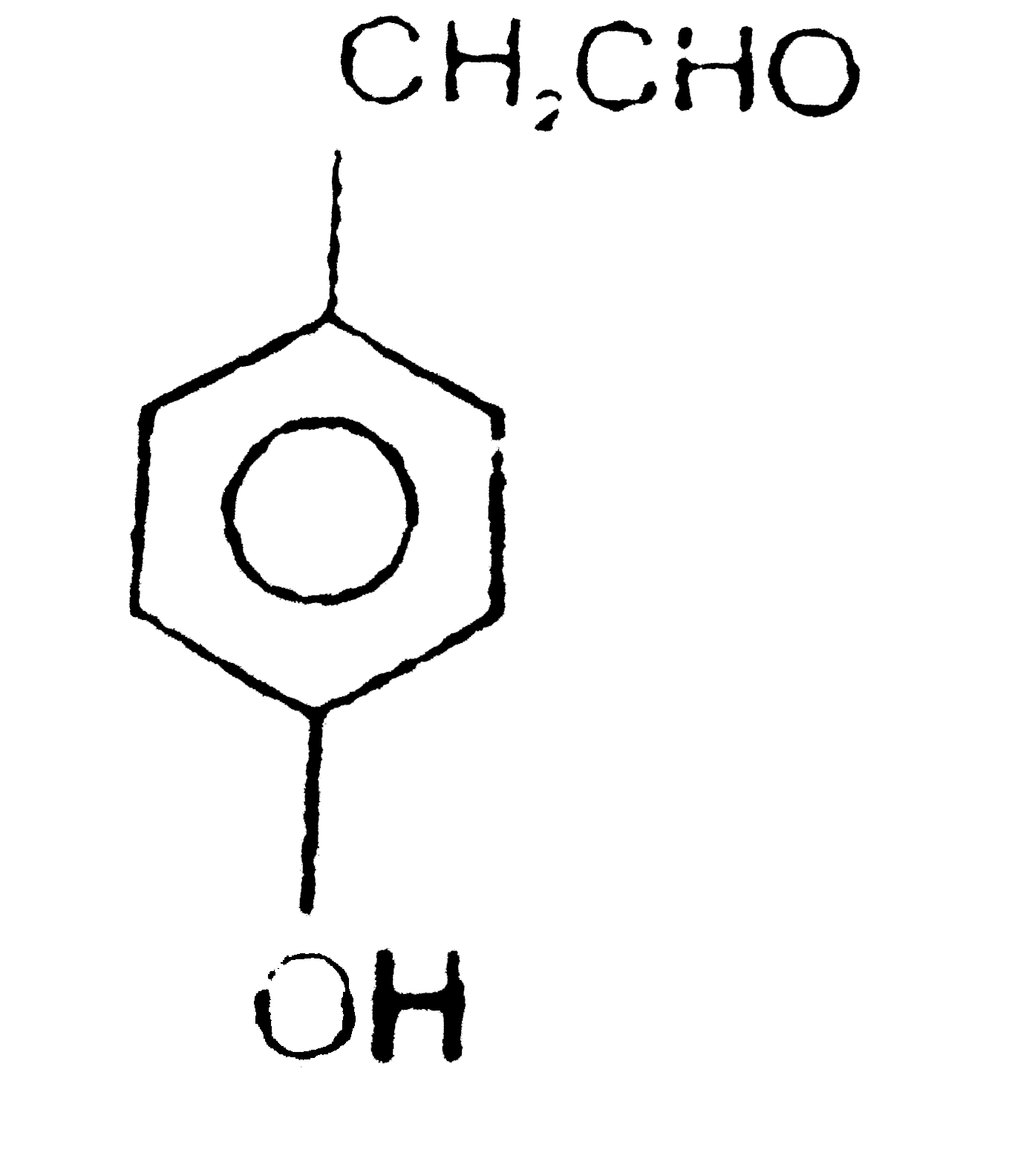
View SolutionA hydrocarbon (R) has six membered ring in which there is no unsaturation.Two alkyl group are attached to the ring adjacent
to each other.One group has 3 carbon atoms with branching at 1st carbon and another has 4 carbon atoms.The larger alkyl group has main chain of three carbon atoms of which second carbon is substituted. Correct IUPAC name of compound (R) is
View SolutionA buffer solution can be prepared from a mixture of
View SolutionIn purification of bauxite ore, it is mixed with coke and heated at 1800∘C in presence of nitrogen, this is :
View SolutionWhich of the following IUPAC name is correct ?
View SolutionWhich of the following IUPAC name is correct?
View SolutionWhich is correct IUPAC name of the following ?
View SolutionWhich of the following IUPAC name is/are correct ?
View SolutionWhich of the following IUPAC names are correct?
View SolutionWhich of the following is correct IUPAC name ?
View SolutionWhich of the following is/are not the correct IUPAC name ?
View SolutionWhich of the following is the correct IUPAC name ?
View SolutionWhich of the following IUPAC names is correct ?
View SolutionWhich of the following IUPAC names are correct.
View SolutionWhich of the following IUPAC names are correct.
View Solution
