When CaCO3 is heated to a high temperature, it undergoes decomposition into CaO and CO2 whereas it is quite stable at room temperature. The most likely explanation of its is
The correct Answer is:CH3CH2COOH(Propanoic acid)→(A); CH3COOCH3(Methyl acetate)→(B) or HCOOC2H5
Step by step video solution for When CaCO_(3) is heated to a high temperature, it undergoes decomposition into CaO and CO_(2) whereas it is quite stable at room temperature. The most likely explanation of its is by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- STRUCTURAL IDENTIFICATIONBOOK - RESONANCE ENGLISHCHAPTER - STRUCTURAL IDENTIFICATIONEXERCISE - Exercise-1 (Part-II)
36 Videos
- STRUCTURAL IDENTIFICATIONBOOK - RESONANCE ENGLISHCHAPTER - STRUCTURAL IDENTIFICATIONEXERCISE - Exercise - 2 (Part-I)
10 Videos
- STRUCTURAL IDENTIFICATIONBOOK - RESONANCE ENGLISHCHAPTER - STRUCTURAL IDENTIFICATIONEXERCISE - Advanced level Problems (Part-III)
12 Videos
- STEREOISOMERISMBOOK - RESONANCE ENGLISHCHAPTER - STEREOISOMERISMEXERCISE - EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))
23 Videos
- SURFACE CHEMISTRYBOOK - RESONANCE ENGLISHCHAPTER - SURFACE CHEMISTRYEXERCISE - Section - 5
1 Video
Similar Questions
Gibbs Helmholtz equation relates the enthalpy, entropy and free energy change of the process at constant pressure and temperature as
ΔG=ΔH−TΔS (at constant P, T)
In General the magnitude of ΔH does not change much with the change in temperature but the terms TΔS changes appreciably. Hence in some process spontaneity is very much dependent on temperature and such processes are generally known as entropy driven process.
When CaCO3 is heated to a high temperature it decomposes into CaO and CO2, however it is quite stable at room temperature. It can be explained by the fact that
View SolutionA: H2 when allowed to expand at room temperature it causes heating effect.
R: H2 has inversion-temperature much below room temperature.
View Solution
Knowledge Check
Wolff rearrangement
When α-Diazoketones are photo-irradiated or heated at high temperature or reacted with silver oxide or silver salts at room temperature, they loose nitrogen and rearrange to form ketene.
The ketenes reacts rapidly with water, alcohol and amines. Therefore, the reactions called Wolff-rearrangement.
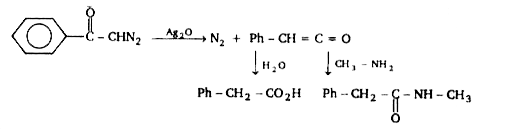
 Product (A) is : A
Product (A) is : A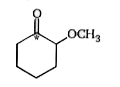 B
B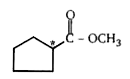 C
C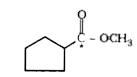 D
D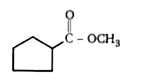
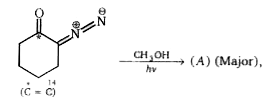
Wolff rearrangement
When α-Diazoketones are photo-irradiated or heated at high temperature or reacted with silver oxide or silver salts at room temperature, they loose nitrogen and rearrange to form ketene.
The ketenes reacts rapidly with water, alcohol and amines. Therefore, the reactions called Wolff-rearrangement.
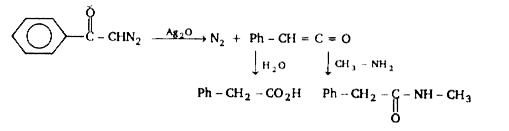
 Major product of the reaction is : A
Major product of the reaction is : A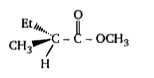 B
B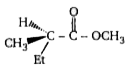 C
C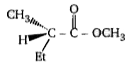 DNone of these
DNone of theseWolff rearrangement
When α-Diazoketones are photo-irradiated or heated at high temperature or reacted with silver oxide or silver salts at room temperature, they loose nitrogen and rearrange to form ketene.
The ketenes reacts rapidly with water, alcohol and amines. Therefore, the reactions called Wolff-rearrangement.
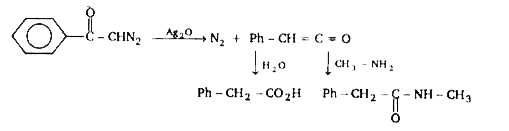
 , Product (A) is : A
, Product (A) is : A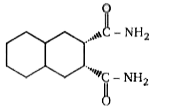 B
B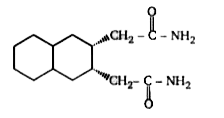 C
C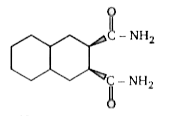 D
D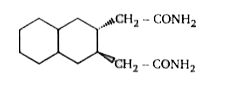
Why CO2 is removed when CaCO3 is heated in a rotary kiln?
View SolutionExplain, why is CO2 a gas at room temperature but SiO2 is a high melting solid.
View SolutionA thin wire of length l when heated to a certain temperature increases its length by 1%. A sheet of the same material of area 2l×l is heated to the same temperature then percentage increase in area will be :-
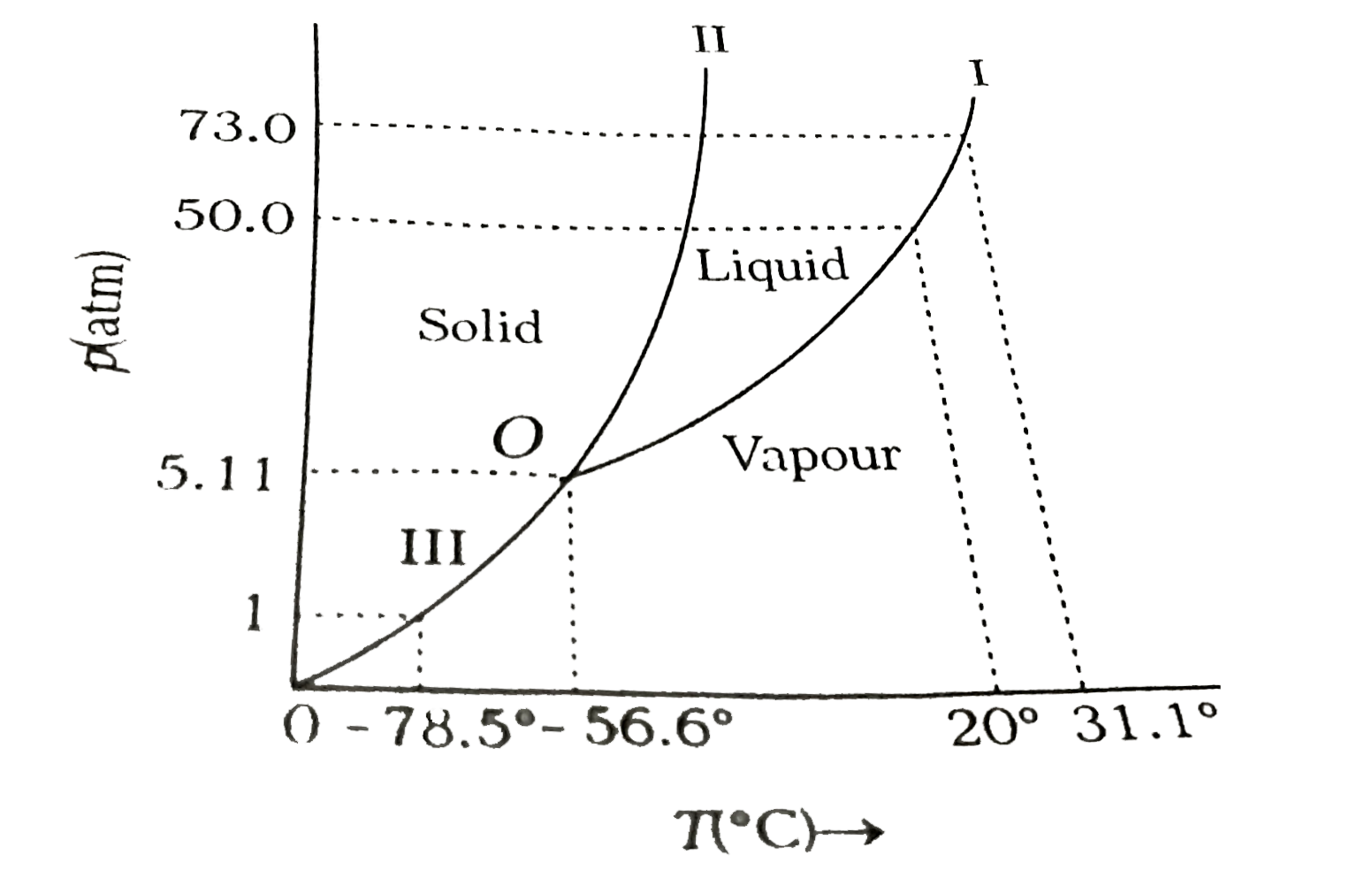
View SolutionAnswer the following questions based on the P – T phase diagram of CO2:
(a) CO2 at 1 atm pressure and temperature–60∘C is compressed isothermally. Does it go through a liquid phase ?
(b) What happens when CO2 at 4 atm pressure is cooled from room temperature at constant pressure ?
(c) Describe qualitatively the changes in a given mass of solid CO2 at 10 atm pressure and temperature –65∘C as it is heated up to room temperature at constant pressure.
(d) CO2 is heated to a temperature 70∘C and compressed isothermally. What changes in its properties do you expect to observe ?
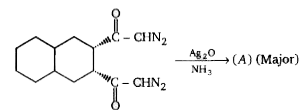
View SolutionWolff rearrangement
When α-Diazoketones are photo-irradiated or heated at high temperature or reacted with silver oxide or silver salts at room temperature, they loose nitrogen and rearrange to form ketene.
The ketenes reacts rapidly with water, alcohol and amines. Therefore, the reactions called Wolff-rearrangement.
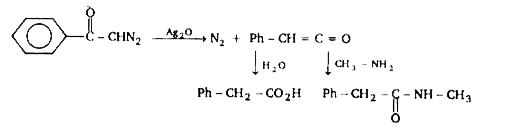
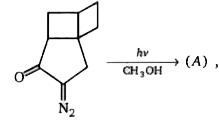 , Product (A) is :
, Product (A) is :
View Solution

