On mixing a certain alkane with chlorine and irradiating it with UV light, it form only two monochloro alkanes. The alkane could be:
propane
pentane
isopentane
neopentane
The correct Answer is:D
The numberof monohalogenation products obtained from any alkane depends upon the number of different types of hydrogen it contains. Compound containng only one type of hydrogen gives only one monohalogenation product.
CH3CH2CH3propane−two types of hydrogen(two monohalogenation structural product)CH3CH2CH2CH3pentane−three types of hydrogen(two monohalogenation structural product)CH3−CH3∣CH−CH2−CH3isopentane−four types of hydrogen (four monohalogenation structural product)H3C−CH3∣C∣CH3−CH3neopentane−one types of hydrogen (one monohalogenation structural product)
Thus the given alkane should be beopentane.
Topper's Solved these Questions
- STRUCTURAL IDENTIFICATIONBook:RESONANCE ENGLISHChapter:STRUCTURAL IDENTIFICATIONExercise:Advanced level Problems (Part-I)
Explore 28 Videos
- STRUCTURAL IDENTIFICATIONBook:RESONANCE ENGLISHChapter:STRUCTURAL IDENTIFICATIONExercise:Advanced level Problems (Part-II)
Explore 18 Videos
- STRUCTURAL IDENTIFICATIONBook:RESONANCE ENGLISHChapter:STRUCTURAL IDENTIFICATIONExercise:Exercise-3 Jee (Advanced) (Part-I)
Explore 2 Videos
- STEREOISOMERISMBook:RESONANCE ENGLISHChapter:STEREOISOMERISMExercise:EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))
Explore 23 Videos
- SURFACE CHEMISTRYBook:RESONANCE ENGLISHChapter:SURFACE CHEMISTRYExercise:Section - 5
Explore 1 Video
Similar Questions
Degree of unsaturation of product form after complete hydrogenation of the following compound:
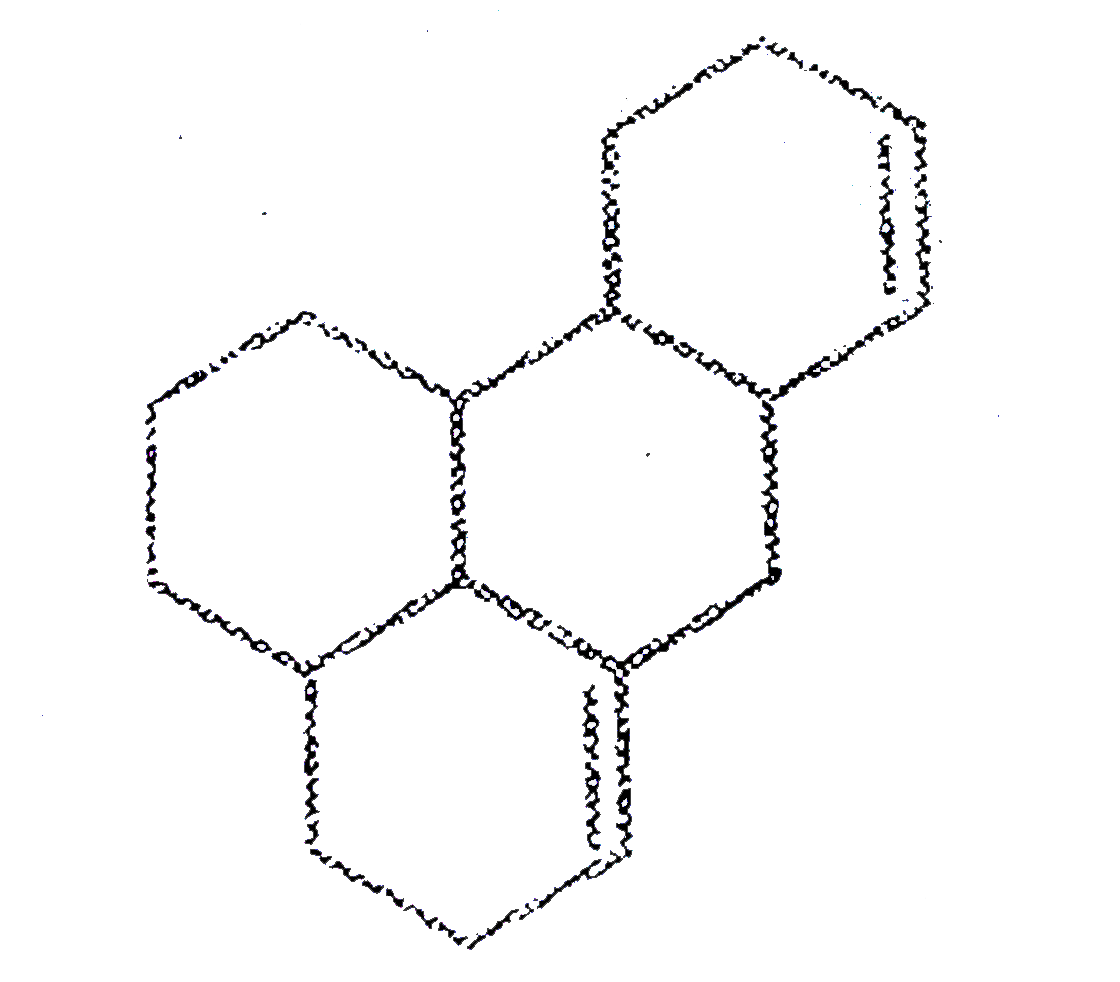
View SolutionA hydrogen on oxidative ozonlysis produces Oxalic acid and Butanedioic acid. Its structrure is
View SolutionFour isomeric para-disubstituted aromatic compounds A to D with molecular formula C8H8O2 were given for identification. Based on the following observations, give structures of the compounds.
View Solution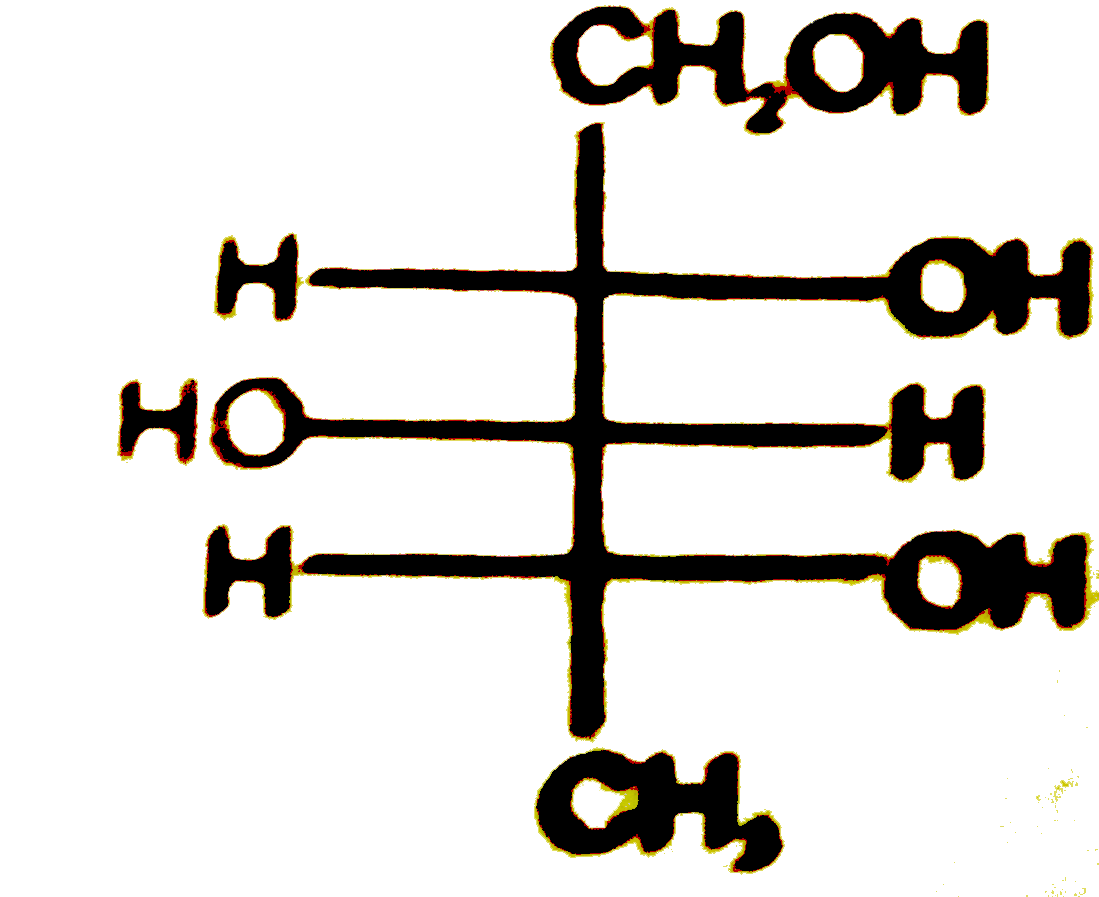 is a Fischer projection of one of_______ stereoisomers ?
is a Fischer projection of one of_______ stereoisomers ?
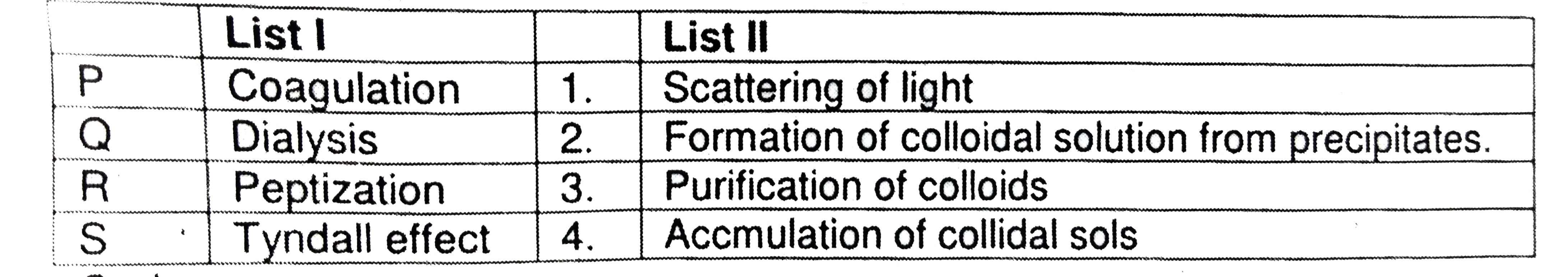
View SolutionMatch list I with list II and select the correct answer :
View SolutionOn mixing certain alkane with chlorine and irradiating it with ultravilet light, it forms only one monochloroalkane. The alkane is
View SolutionOn mixing a certain alkane with chlorine and irradiating it with ultraviolet light, it forms only one monochloroalkane this alkane could be :
View SolutionOn mixing a certain alkane with chlorine and irradiating it with ultraviolet light, it forms only one monochloroalkane this alkane could be :
View SolutionOn mixing an alkane with chlorine and irradiating with ultra violet light, it forms only one monochloro alkane. The alkane is
View SolutionOn mixing certain alkane with chlorine and irradiating it with ultravilet light, it forms only one monochloroalkane. The alkane is
View SolutionOn mixing a certain alkane with chlorine and irradiating it with ultraviolet light, it forms only one monochloroalkaane. This alkane could be
View SolutionOn mixing certain alkane with chlorine and irradiating it with Ultravioet light , it forms only one monochloro alkane .
the alkane xould be :
View SolutionOn mixing an alkane with chlorine and irradiating with ultra-violet light, it forms only one mono-chloroalkane. The alkane is
View Solutionएक एल्केन की अभिक्रिया Cl2 से UV प्रकाश की उपस्थिति में कराने पर, केवल एक मोनोक्लोरो एल्केन बनती है तब वह एल्केन होगी
View SolutionOn mixing a certain alkane with chlorine and irradiating it with ultraviolet light, it forms only one monochloroalkane. This alkane should be
View SolutionOn mixing certain alkane with chlorine and irradiating it with ultraviolet light ,one forms only one monochloro alkane .The alkane could be :
View Solution
