Which of the following compounds after complete hydrogenation will form three monochloro structural isomeric products ?
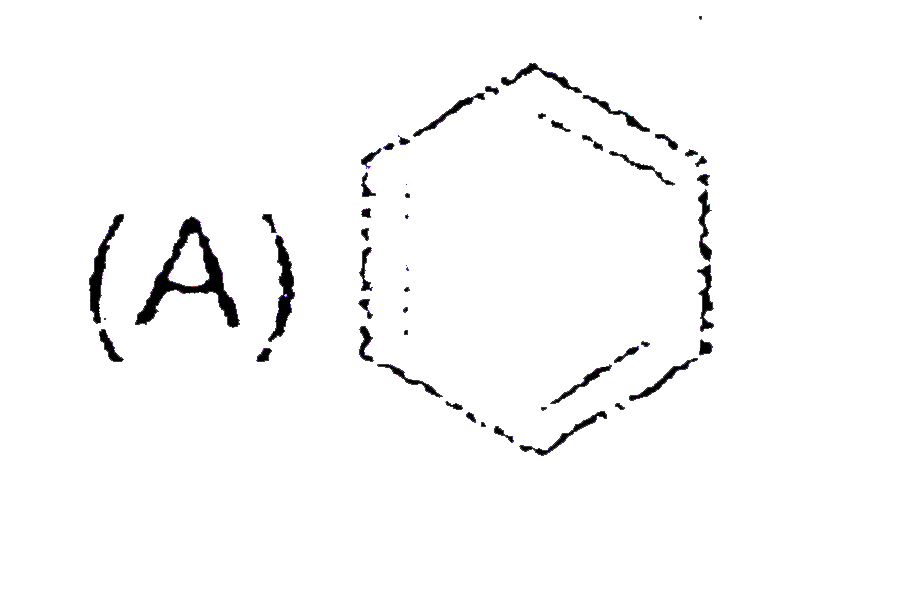
CH3−CH∣CH3−C∣∣CH2−CH3

HC≡C−C≡CH∣CH−C≡CH
The correct Answer is:C, D
To determine which compound will form three monochloro structural isomeric products after complete hydrogenation, we can follow these steps:
Step 1: Identify the Compound
We need to identify the compound from the options provided that, upon complete hydrogenation, will yield a saturated hydrocarbon (alkane) with the potential for three distinct monochloro derivatives.
Step 2: Perform Complete Hydrogenation
Complete hydrogenation of an unsaturated compound (like an alkene or alkyne) converts it into a saturated compound (alkane). For example, if we have a compound like an alkene, it will convert to hexane (C6H14) after hydrogenation.
Step 3: Structure of the Resulting Alkane
After hydrogenation, we will have a straight-chain alkane. For instance, if the compound is 1-hexene, the resulting product after hydrogenation will be hexane (C6H14).
Step 4: Identify Possible Monochloro Derivatives
Now we need to identify the positions where chlorine can be substituted on the hexane molecule. The positions are as follows:
- Chlorine can be substituted at the 1st carbon (C1).
- Chlorine can be substituted at the 2nd carbon (C2).
- Chlorine can be substituted at the 3rd carbon (C3).
Step 5: Check for Structural Isomerism
When chlorine is substituted at C1, C2, and C3, we need to check if these substitutions yield unique structural isomers:
1. 1-chlorohexane (Cl at C1)
2. 2-chlorohexane (Cl at C2)
3. 3-chlorohexane (Cl at C3)
Substituting chlorine at C4 would yield a compound that is structurally identical to 2-chlorohexane due to symmetry, and substituting at C5 would yield a compound that is identical to 1-chlorohexane. Thus, we only have three distinct structural isomers.
Conclusion
The compound that, after complete hydrogenation, forms three monochloro structural isomeric products is option C.
---
We need to identify the compound from the options provided that, upon complete hydrogenation, will yield a saturated hydrocarbon (alkane) with the potential for three distinct monochloro derivatives.
Complete hydrogenation of an unsaturated compound (like an alkene or alkyne) converts it into a saturated compound (alkane). For example, if we have a compound like an alkene, it will convert to hexane (C6H14) after hydrogenation.
After hydrogenation, we will have a straight-chain alkane. For instance, if the compound is 1-hexene, the resulting product after hydrogenation will be hexane (C6H14).
Now we need to identify the positions where chlorine can be substituted on the hexane molecule. The positions are as follows:
- Chlorine can be substituted at the 1st carbon (C1).
- Chlorine can be substituted at the 2nd carbon (C2).
- Chlorine can be substituted at the 3rd carbon (C3).
When chlorine is substituted at C1, C2, and C3, we need to check if these substitutions yield unique structural isomers:
1. 1-chlorohexane (Cl at C1)
2. 2-chlorohexane (Cl at C2)
3. 3-chlorohexane (Cl at C3)
The compound that, after complete hydrogenation, forms three monochloro structural isomeric products is option C.
টপার এই প্রশ্নগুলো সমাধান করেছে
- STRUCTURAL IDENTIFICATIONBook:RESONANCE ENGLISHChapter:STRUCTURAL IDENTIFICATIONExercise:Advanced level Problems (Part-III)
Explore 12 Videos
- STRUCTURAL IDENTIFICATIONBook:RESONANCE ENGLISHChapter:STRUCTURAL IDENTIFICATIONExercise:Advanced level Problems (Part-I)
Explore 28 Videos
- STEREOISOMERISMBook:RESONANCE ENGLISHChapter:STEREOISOMERISMExercise:EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))
Explore 23 Videos
- SURFACE CHEMISTRYBook:RESONANCE ENGLISHChapter:SURFACE CHEMISTRYExercise:Section - 5
Explore 1 Video
সম্পর্কিত প্রশ্ন
What simple laboratory test could be performed to distinguish between 1-pentyne and 2-pentyne?
View SolutionDegree of unsaturation of product form after complete hydrogenation of the following compound:
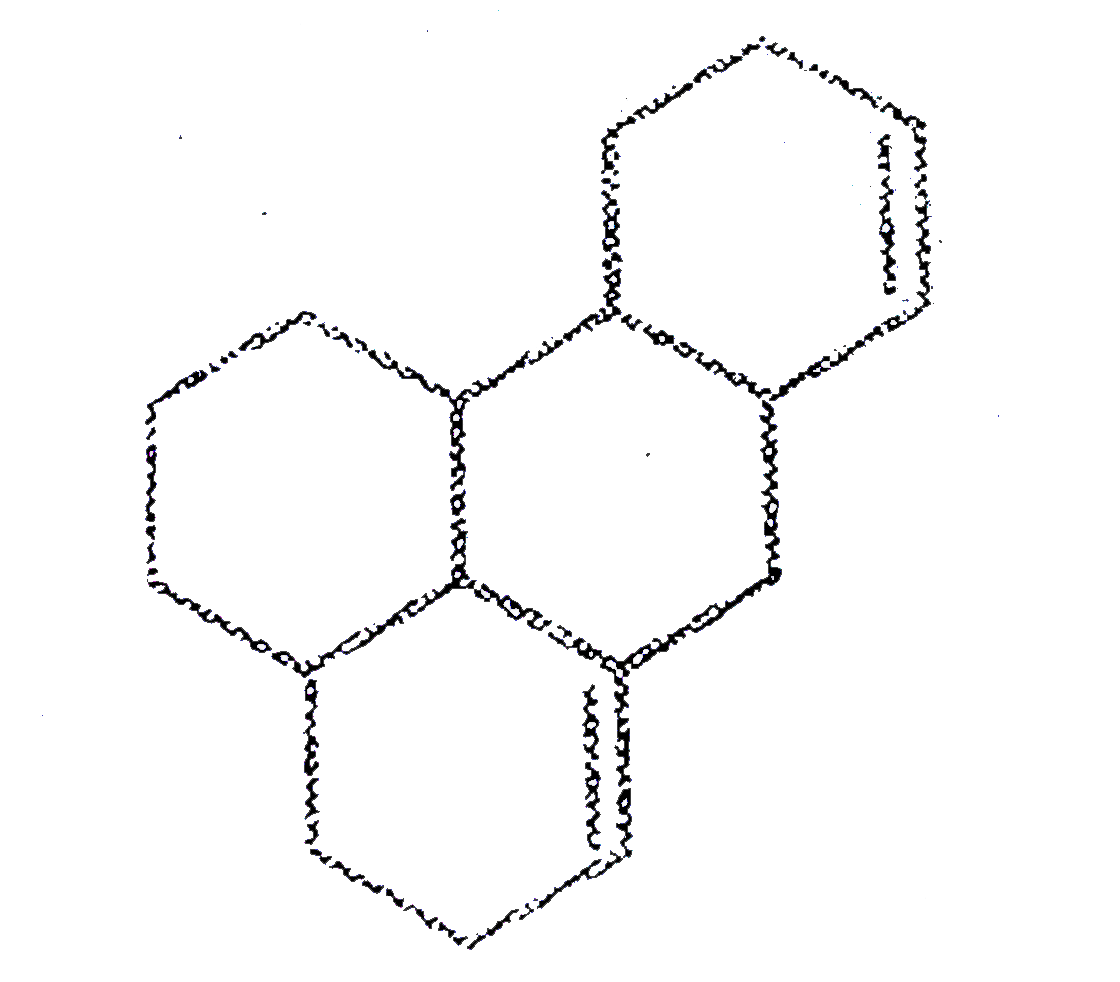
View Solution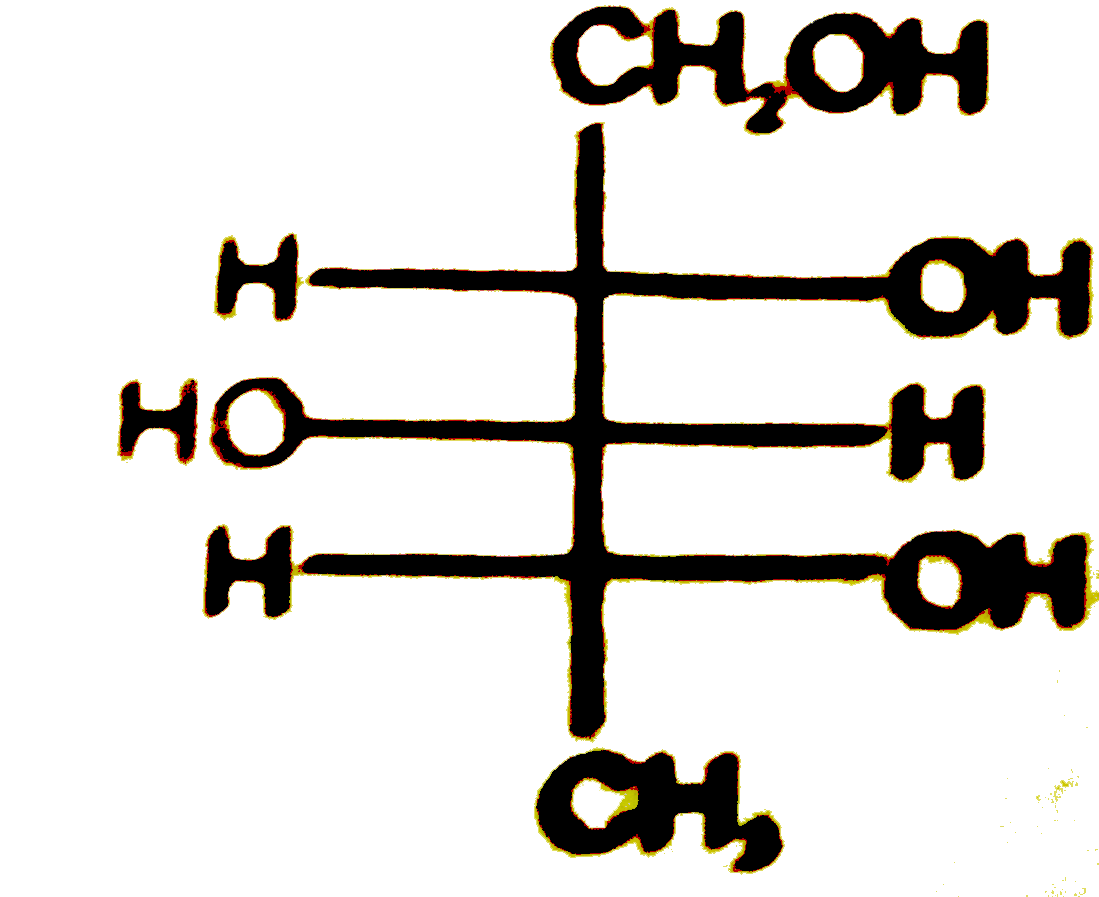 is a Fischer projection of one of_______ stereoisomers ?
is a Fischer projection of one of_______ stereoisomers ?
View SolutionMatch list I with list II and select the correct answer :
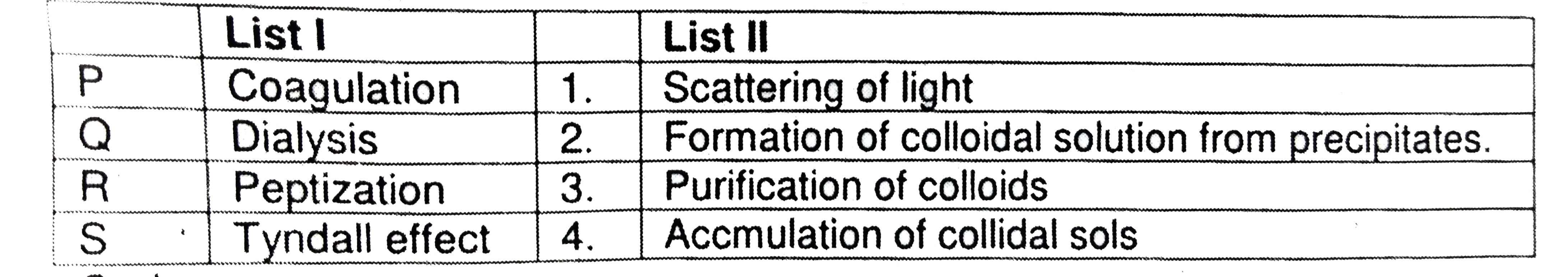
View SolutionHow many monochloro structural isomeric products are formed when isohexane is treated with CI2/hv :
View Solution
Which of the following set gives the product that give two monochloro structural products after reaction with Cl/hv ?
View SolutionWhich of the following alkene gives four monochloro (structural isomer) products after hydrogenation ?
View SolutionWhich of the following compound will give four monochloro (structural) product on monochlorination.
View SolutionDegree of unsaturation of product form after complete hydrogenation of the following compound:
View SolutionWhich of the following compounds after complete hydrogenation will form three monochloro structural isomeric products ?
View SolutionWhich of the following alkene gives four monochloro (structural isomer) products after hydrogenation ?
View SolutionWhich of the following compound will give four monochloro (structural) product on monochlorination.
View SolutionWhich of the following compounds after complete hydrogenation will form three monochloro structural isomeric products ?
View Solutionनिम्नलिखित किस यौगिक के केवल दो समावयवी मोनोक्लोरो प्रतिस्थापन यौगिक संभव है?
View SolutionWhich of the following compound has only two isomeric monochloro derivates?
View Solutionनिम्न में से कौनसा यौगिक पूर्णतयाः हाइड्रोजनीकरण के दौरान तीन मोनोक्लोरो संरचनात्मक उत्पाद देगा।
View Solution
